Abstract
Acute Respiratory Distress Syndrome (ARDS) is a common entity in critical care medicine and associated with many diagnoses, including trauma and sepsis, which may lead to multiple organ failure and death. Pathophysiologically, increased capillary permeability is the hallmark of ARDS which is characterized by damage of the capillary endothelium and alveolar epithelium in association with impaired fluid removal from the alveolar space and the accumulation of protein-rich fluid inside the alveoli. The clinical management of patients with ARDS is even more difficult, because in the presence of capillary leakage in the lungs, adequate intravascular volume and cardiac preload are required to maintain organ perfusion. The amount of pulmonary edema fluid is, however, difficult to determine at the bedside. Pulmonary edema can be detected on physical examination and may be confirmed by chest radiography. However, it has been shown to be difficult to quantify the extent of pulmonary edema based on chest radiography or other non-invasive measures. The transpulmonary thermo-dye dilution technique has been introduced as an instrument to quantify the fluid in the pulmonary capillary bed, i.e., extravascular lung water (EVLW). This technique as shown to be potentially valuable in the management of critically ill patients and has been further developed to be clinically available nowadays as single transpulmonary thermodilution. The following review deals with the measurement of EVLW and its place in the management of critically ill patients with ARDS.
Introduction
Acute Respiratory Distress Syndrome (ARDS) which was initially described in 1967 by Ashbaugh et al. [1] is a common entity in critical care medicine. ARDS is associated with many diagnoses, including trauma and sepsis, and may lead to multiple organ failure with high mortality. Pathophysiologically, increased capillary permeability is the hallmark of ARDS which is characterized by damage of the capillary endothelium and alveolar epithelium in association with impaired fluid removal from the alveolar space and the accumulation of protein-rich fluid inside the alveoli.
The clinical management of patients with ARDS is even more difficult, because in the presence of capillary leakage in the lungs, adequate intravascular volume and cardiac preload are required to maintain organ perfusion. The amount of pulmonary edema fluid is, however, difficult to determine at the bedside. Pulmonary edema can be detected on physical examination by the presence of rales and may be confirmed by chest
radiography and the presence of bilateral pulmonary opacities. However, it has been shown to be difficult to quantify the extent of pulmonary edema based on chest radiography or other non-invasive measures [2]. In the 1980’s Sibbald et al. [3] studied the transpulmonary thermo-dye dilution technique as an instrument to quantify the fluid in the pulmonary capillary bed, i.e., extravascular lung water (EVLW). Over the following years this technique as shown to be potentially valuable in the management of critically ill patients [4,5] and has been further developed [6,7] to be clinically available nowadays as single transpulmonary thermodilution. The following review deals with the measurement of EVLW and its place in the management of critically ill patients with ARDS.
Measurement of extravascular lung water
Historically, the so-called transpulmonary double (i.e., thermo-dye) dilution technique was used for the clinical measurement of EVLW. In this technique two different indicators with specific properties and differences are simultaneously injected central venously and were then detected not prior to, but after passing through the lungs in the arterial system (transcardiopulmonary) by an appropriate sensor. In clinical practice, a bolus of cooled (0- 4°C) indocyanine green (ICG) was used and an arterial thermistor-fiberoptic catheter was used. In most cases, femoral arterial catheterization, which has been shown to be safe, was performed for measurement of the EVLW [8]. In contrast to the dye, which immediately binds to plasma proteins and remains fully in the intravascular bed, the cold equilibrates with extravascular structures. The dye (ICG) allows assessment of the intravascular compartment, i.e., the intrathoracic blood volume (ITBV). For each indicator, the concentration between both volumes is called the extravascular lung water: EVLW = ITTV - ITBV. While ITBV can be used as a cardiac preload parameter, EVLW is a marker of extravascular fluid in the lungs.
Notably, for several years now ultrasound techniques have also been suggested for assessing EVLW since Blines as vertical artifacts and arising comets were found in the presence of interstitial-alveolar imbibition [9]. These authors found a linear correlation between comet score, a sign of extravascular lung water, and radiologic lung water score (r= 0.78) while intra-patient variations showed an even stronger correlation between changes in both variables (r= 0.89). They concluded that the comet-tail is a simple, non-time-consuming, and reasonably accurate chest ultrasound which can be obtained at bedside by ultrasound. More recently, the same group compared ultrasound with EVLW in an animal model [10]. In a pig model of ALI/ARDS, B-lines assessed by lung ultrasound provided a simple, semi-quantitative, noninvasive index of lung water accumulation which
strongly correlated to invasive gravimetric assessment. Clinical data [11] supports lung ultrasound as an appropriate method with which to assess EVLW. Recently, Lichtenstein et al. [12] suggested the FALLSprotocol in the management of patients with shock where simple echocardiography is used to rule out obstructive shock (tamponade, pulmonary embolism) thereafter the lung is investigated. In fact, fluid is administered until signs of extravasation in the lungs (B-lines) occur, demanding cessation of fluid therapy.
However, this sequential approach that is combined with the usual clinical, biochemical and echocardiographic parameters is not widely accepted and must undergo validation in adequate studies. Furthermore, several other techniques (e.g., computed tomography, magnetic resonance tomography, positron emission tomography and bioimpedance) [13-16] have been used to quantify EVLW. However, the following review will focus on transpulmonary indicator dilution for measurement of EVLW.
Technology assessment
By using the double-indicator technique, Sibbald et al. [17] showed the difference between cardiac and noncardiac pulmonary edema. Patients with ARDS were shown to have a higher EVLW while their hydrostatic component (i.e., pulmonary artery occlusion pressure) was much lower (“permeability edema”) [17]. For a given hydrostatic pressure, EVLW was much higher in patients with a permeability induced lung edema (ARDS) when compared to “pure” hydrostatic pathophysiology (“cardiogenic edema”). The change in hydrostatic pressure could be demonstrated to be associated with a less steep increase in EVLW when compared to ARDS patients. Similar findings had been described before in an animal experimental setting [18]. Furthermore, for similar chest X-ray scores, EVLW in patients with ARDS was significantly higher than in cardiac patients. Consequently, EVLW cannot be estimated reliably from the hydrostatic component in ARDS this reference, only 65% of patients with fulfilled ARDS criteria had an EVLW >7 ml/kg. Consequently, about one third of patients with ARDS do not have pulmonary edema, as assessed by EVLW from the transpulmonary double indicator dilution technique.
Although effective at the bedside, the double-indicator (cooled ICG) technique is relatively time consuming, cumbersome and expensive. An approach which provides circulatory volumes and EVLW from a singleindicator technique using arterial thermodilution alone would be an advantage. Estimation of EVLW by single thermodilution is based on the assumption that, for several mixing chambers in a series with identical flow, the
decay of the dilution curve is predominantly determined by the largest compartment [20]. In an animal study, Neumann et al. [21] showed that EVLW could be reliably derived from thermodilution alone. Shortly afterwards, our group derived a correction factor for critically ill patients which was then validated in 209 other ICU patients [7]. In detail, thermodilution derived EVLW was correlated with double indicator derived EVLW
by y=0.83*x + 1.6 ml/kg, r = 0.96, P < 0.0001. Bias between both techniques was 0.2 ml/kg with a standard deviation of 1.4 ml/kg. Thermodilution EVLW systematically overestimated EVLW at low-normal values underestimated EVLW at higher values (>12 ml/kg). Changes between the first two time points of simultaneous measurements were also analyzed and revealed r=0.87.
The reliability of EVLW by transpulmonary thermodilution was studied in a swine model by FernándezMondéjar et al. [22] who reported on EVLW measurements before and immediately after intratracheal instillation of normal saline. In normal and edematous lungs EVLW increased and the transpulmonary thermodilution technique was described to accurately detect small increases in EVLW and so may permit accurate diagnosis of incipient pulmonary edema. EVLW as measured by the single thermodilution technique has been shown to be nearly identical to that measured by the double indicator technique but with a slight overestimation when EVLW is normal (<7 ml/kg) [23]. In the clinically more relevant pathological range, a close agreement between both techniques was found with high reproducibility, emphasizing that single thermodilution is accurate enough for the estimation of EVLW in clinical practice [23]. Additionally, in an experimental model of cardiogenic and non-cardiogenic pulmonary edema, thermodilution-derived EVLW was found to closely correlate with gravimetry [24].
In general, the accuracy and reproducibility of EVLW measurements by transpulmonary indicator dilution techniques per se is high [25,26,27]. Noteworthy, recent findings indicate that reliability of EVLW measurement remains preserved when the injection of room temperature saline is used [28]. Michard et al. [29] noted that the estimation of EVLW by transpulmonary thermodilution was influenced by the amount of EVLW, the PaO2/FiO2 ratio, the tidal volume, and the level of positive end-expiratory pressure. However, compared technique for the measurement of EVLW might still be influenced by changes in perfusion and ventilation. In addition, the single transpulmonary thermodilution technique, might require adjustment of the mathematical relationship to the particular condition and species subjected to the measurement.
One more recent concept is that of the pulmonary vascular permeability index (PVPI) which is the ratio between EVLW and the pulmonary blood volume (PBV), i.e. between the extravascular and the intravascular fluid compartments of the lung. The idea is that the capillary barrier in the pulmonary microcirculation is more insufficient when a low ITBV is associated with a high EVLW. In contrast, the barrier is tight and PVPI is low
in the presence of a high ITBV and low EVLW [31]. The PVPI has been studied in animals using the transpulmonary thermodilution technique. The PVPI was higher in pigs with artificial ARDS (oleic acid model) compared to animals with simulated left heart failure (left atrial balloon) [24]. Additionally, this study nicely showed that single thermodilution technique correlated well with gravimetry [24] (figure 1). Also clinical data suggest that indexes of pulmonary permeability provided by transpulmonary thermodilution may be useful for determining the mechanism of pulmonary edema in the critically ill [32]. In this study [32], patients with cardiac edema had a higher PVPI and lower left ventricular ejection fraction than ARDS patients. As such, the PVPI allows differentiation between cardiogenic and non-cardiogenic pulmonary edema, because in noncardiogenic pulmonary edema both EVLW and PBV will be high [24, 32, 33]. Chew et al. [33] showed that the ratio seemed to be a better marker of disease severity in patients with a Lung Injury Score >2.5, implying that patients with severe ARDS have greater lung edema due to greater pulmonary permeability.
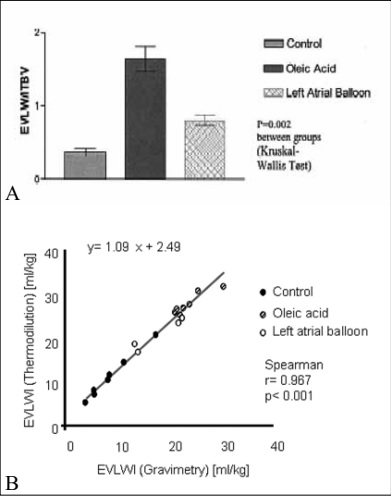
Figure 1. Reliability of single transpulmonary thermodilution technique for the assessment of extravascular lung water
(EVLW) in animals with cardiogenic and non-cardiogenic pulmonary edema.
A Extravascular lung water (EVLW) to intrathoracic blood volume (ITBV) ratio in the three different groups: control group, oleic acid group (increased permeability pulmonary edema), and left atrial balloon group (hydrostatic pulmonary edema; mean ± SD).
B Correlation of extravascular lung water index (EVLWI) as measured by single transpulmonary thermodilution compared with gravimetric measurement. From [24] with permission from the authors.
In a human study, Tagami et al. [34] evaluated the correlation between pre-mortem EVLW value by single transpulmonary thermodilution and post-mortem lung weight from 30 autopsies completed within 48 hours after final clinical measurement. EVLW correlated closely with post-mortem lung weight (r= 0.90). The normal EVLW values indexed by predicted body weight were approximately 7.4 ± 3.3 mL/kg (7.5 ± 3.3 mL/kg for males and 7.3 ± 3.3 mL/kg for females). More recently Eichhorn et al. [35] published a meta-analysis in which they found that mean EVLW was 7.3 ml/kg in surgical patients and 11 ml/kg in non-surgical septic patients, respectively. In the septic group all studies except one showed EVLWI values above the limit of 7 ml/kg (20/21), whereas 9 of the 19 studies including surgical patients reported normal values of <7 ml/kg. Clinical studies show that EVLW may exceed values of up to about 40 ml/kg [35] in severe cases.
EVLW reflects the lung water that is contained in the perfused areas of the lungs (the distribution volume of the cold indicator). Thus the measured EVLW may be underestimated when significant lung areas are excluded from circulation, as in massive pulmonary embolism, a very low cardiac output, or with high PEEP [36,37]. However, these theoretical considerations have only a marginal practical value as shown by Phillips et al. [38],
seriously ill patients, and yet the EVLW was exceedingly high in spite of this high level of non-perfusion. Although surgical lung resection may constitute a limitation in the estimation of EVLW by thermodilution [39,40], changes in thermodilution-derived EVLW may be nevertheless helpful in the clinical management. Furthermore, heterogeneity in pulmonary edema and perfusion may substantially influence the accuracy of EVLW measurement using these techniques.
Notably, postmortem gravimetry used as reference technique for measurement of EVLW also has its limitations [41,42]. Furthermore, comparison of gravimetrically determined EVLW with that by other techniques may be influenced by the time elapsing from euthanasia to lung removal, and by changes in distribution of pulmonary blood following cardiac arrest. In detail, gravimetry may underestimate the real value of EVLW due to partial
re-absorption of edema fluid before excision of the lungs. In summary, single and double indicator dilution techniques may yield relevant clinical information on EVLW which make them potentially promising for their application at the bedside of critically ill patients.
Clinical considerations
Clinical examination, chest X-ray, and blood gases have been proven to be of only limited significance for quantifying pulmonary edema [43-45]. With respect to chest X-ray, scoring systems have been suggested for quantifying EVLW. Although there is a correlation with EVLW in terms of higher scoring values and extent of EVLW as measured by the double-indicator dilution technique [46], there is wide range of scatter and changes
which are obviously not always identified correctly. Furthermore, chest X-ray is not time effective, with delays of several hours for detecting EVLW [46].
An increase in EVLW is associated with reduced lung compliance, increased venous admixture, and arterial hypoxemia, causing mortality in excess of up to 40% [43, 47]. In general, positive fluid balances in critically ill patients with enhanced risk of extravasation of fluids have been found to increase mortality [48, 49]. Correspondingly, fluid restriction in order to counteract pulmonary edema, has been described as positively influencing the course of illness and improving outcomes [4,5].
Thus far EVLW has been shown to be the best pulmonary specific index of disease severity and predictor of outcome available in patients with ALI/ARDS [50,51], while all other clinical methods for quantifying lung water are either insensitive or non-specific (e.g., chest X-ray, oxygenation). In our own retrospective analysis of 373 critically ill patients, the mortality rate was 65% in patients with an EVLW>15 ml/kg and only 33% in
patients with an EVLW <10 ml/kg [26] (figure 2). In detail, the maximum EVLW was significantly higher in non-survivors (n= 186) than in survivors (n= 187) [median, 14.3 mL/kg vs. 10.2 mL/kg, respectively. In a univariate logistic regression model, the EVLW at baseline as well as SAPS II (Simplified Acute Physiology Score) and APACHE II (acute physiology and chronic health evaluation) scores were significant predictors of mortality. Correlation increased if the SAPS II and APACHE II scores were combined, but the improvement with SAPS II alone was not significant. The addition of baseline EVLW further increased the value, indicating that EVLW contributes independently to prognosis.
.jpg)
Figure 2. Extravascular lung water (EVLW) and outcome in 373 critically ill patients of a mixed non-cardiosurgical
operative ICU. Box plots for different sub-populations of patients (i.e., sepsis, ARDS, all others). Bold lines
indicate medians, box plots indicate 25-75 th percentiles, and bars indicate the 1.5 fold of the whole box length.
Circles indicate values between 1.5-fold to threefold of whole length, and outliers (outside of the whole box
length) are indicated by asterisks. The bold asterisk indicates statistical significance (Mann-Whitney-U test).
From [26] with permission from the authors.
An increased EVLW value on day 1 after trauma, indexed to the predicted (instead of actual) body weight, was found to identify patients who developed sepsis later on [52]. EVLW indexed to predicted body weight (EVLWp), EVLW, and Vd/Vt, but not P/F ratio were able to discriminate between survivors and non-survivors. Three-day average EVLWp >16 mL/kg predicted in-hospital mortality with 100% specificity and 86% sensitivity [52]. Thus, increased EVLW is a feature of early ARDS and predicts survival. Indexing EVLW to the predicted body weight, instead of actual body weight was found to improve the predictive value of EVLW for survival and correlates with markers of disease severity.
Furthermore, thermodilution derived EVLW correlated with the severity of sepsis-induced acute lung injury [53]. In a prospective, observational study, Kuzkov et al. [53] described how EVLW demonstrated a moderate correlation with markers of acute lung injury, such as lung compliance, oxygenation ratio, roentgenogram quadrants, and lung injury score. In non-survivors, EVLWI and permeability indexes were significantly increased on day 3. Thus, EVLW may be of value as an indicator of prognosis and severity of sepsis-induced acute lung injury. Of particular importance for the clinician, measurement of EVLW is not influenced by pleural effusion as could be shown in patients undergoing thoracocentesis [54]. Notably, drainage of largevolume effusions resulted in a statistically significant increase in thermodilution derived EVLWI. Thus, pleural effusions do not take part in single-indicator TPTD as a part of the pulmonary thermovolume and do not increase TPTD-derived EVLWI [55]. Furthermore, reliability of EVLW is preserved during running venovenous renal replacement therapy [56], i.e., the extracorporeal circuit does not need to be interrupted in order to do the measurements. As applied in patients with an ARDS and therefore of particular clinical relevance, also influence of extracorporeal lung assist systems has been studied. By principle, the higher the flow through an extracorporeal system is the higher the loss of indicator and thus cardiac output will be overestimated [57,58].
Likely, a pumpless system (PECLA) with a flow of up to 20% of cardiac output does not reduce the reliability of TPTD derived EVLW [59]. However, further data are required to confirm these preliminary findings. Although the definition of ARDS have been updated very recently [60], EVLW is still not a criterion and patients who are not diagnosed as having ARDS may have increased EVLW [19]. Notably, an increased EVLW (> 7 ml/kg) has been repeatedly suggested as one of the ARDS criteria [61,62]. However, other authors have claimed that this still has to be prospectively validated and confirmed [63]. Nevertheless, EVLW was very recently shown to predict progression to ALI in patients with risk factors on average 2.6±0.3 days before they fulfilled the conventional criteria, and that this period may represent a missed opportunity for interventions [50].
Extravascular lung water guided treatment
The fundamental question is whether an aggressive approach to reducing the amount of EVLW when guided by EVLW or other clinical parameters can reduce mortality in patients with pulmonary edema. Being an independent predictor of survival, EVLW can present a landmark in the management of critically ill patients requiring fluid and vasoactive drug support.
The measurement of EVLW may play an important role in the fluid management of critically ill patients since a positive fluid balance has been repeatedly shown to be independently associated with worst outcome [48]. Moreover, improved outcome has been shown in critically ill patients when their fluid management was guided by EVLW compared to management guided by the pulmonary artery catheter [5]. More recently it was shown that fluid loading was associated with an increase in EVLW of ≥10% in 21% of critically ill patients [64]. An early diagnosis of the pathological accumulation of EVLW during resuscitation may allow for earlier interventions and considerable changes in the therapeutic plan [64]. Finally, frequent determination of EVLW may identify the point when de-resuscitation should be started, namely, the institution of an aggressive negative fluid balance once the hemodynamic status stabilizes.
In general, patients with acute lung injury may benefit from management guided by EVLW [4,5]. In those patients, there is a need to control EVLW when developing pulmonary edema, massive fluid shifts, and severe changes in microvascular permeability. Such changes are known to occur in numerous critical care conditions, i.e., sepsis, burns, non-cardiogenic (ALI and ARDS) and cardiogenic lung edema, multiple trauma with severe
blood loss, ischemia/ reperfusion injury etc. [65,66]. Thus, in principle, any critical illness associated with shock and tissue hypoperfusion which is refractory to fluid resuscitation is a potential subject for EVLW monitoring, including in children [67]. In such a scenario, management according to the EVLW may help in diagnosing and treating pulmonary edema, especially as protocols exist which emphasize that resolution of lung edema can be hastened.
During sepsis-induced pulmonary edema, the accumulation of EVLW occurs before changes in gas exchange, chest X-ray and eventually pressure variables. Furthermore, the latter variables are non-specific tools and moreover potentially influenced by a variety of factors. Several studies have shown that in sepsis commonly used pressures such as the PAOP and right atrial pressure are poor indicators of lung edema [68]. In contrast to
central venous pressure, EVLW correlates with markers of lung injury, including the oxygenation ratio, lung compliance, roentgenogram quadrants, and lung injury score. Interestingly, the day after the onset of severe sepsis, EVLW correlated negatively with platelet count, indicating a role of platelet sequestration in the development of lung edema. Furthermore, endothelin-1 plasma concentrations which may increase pulmonary microvascular permeability and EVLW were positively correlated [53].
With respect to the prognostic properties of EVLW, as early as the 1980’s Sturm described a stepwise increase in mortality with increasing EVLW [69]. As mentioned above, own data showed that EVLW at baseline, SAPS scores, and APACHE scores were significant predictors of mortality. In particular, patients with ARDS had a significantly higher EVLW (14.9 ml/kg) than other patients. Also, subgroup analysis indicated that in patients
with sepsis, non-survivors had a significantly higher EVLW than survivors [26].
More than two decades ago, Eisenberg et al. [4] had prospectively evaluated a protocol that included EVLW instead of PAOP measurements to guide hemodynamic management of critically ill patients. Patients were randomized to receive either protocol management or routine management group. In the routine management group (RM), EVLW measurements were unknown to the primary care physicians. The 2 groups were similar
with respect to age, gender, and severity of illness. In patients with initially high EVLW, EVLW fell to a greater extent in the protocol management group (PM) than in RM patients (18 ± 5 vs. 4 ±- 8% decrease). This difference was even greater in patients with heart failure. No adverse effects on oxygenation or renal function occurred by following the protocol. Mortality for the groups as a whole were similar, but was significantly better for PM patients with initially high EVLW and normal airway pressures (predominantly patients with sepsis or ARDS). For both groups, patients with an initial EVLW > 14 ml/kg had a significantly greater mortality than those with a lesser EVLW: 13 of 15 (87%) vs. 13 of 32 (41%), p< 0.05. The authors concluded that management based on a protocol using EVLW measurements is safe, may hasten the resolution of pulmonary edema, and may lead to improved outcome in some critically ill patients.
In 1992, Mitchell et al. [5] published a randomized, prospective study to evaluate whether fluid management that emphasized diuresis and fluid restriction in patients with pulmonary edema could affect the development or resolution of EVLW and duration of mechanical ventilation and length of ICU stay in critically ill patients. Pulmonary artery catheterization was performed in 101 patients of whom 52 patients were randomized to an
EVLW management group using a protocol based on bedside indicator-dilution measurements of EVLW. The other 49 patients were randomized to a wedge pressure (WP) management group in whom fluid management decisions were guided by WP measurements. A total of 89 patients had pulmonary edema (defined as EVLW > 7 ml/kg ideal body weight). Except for a clinically unimportant difference in mean age, the two groups were
entirely comparable at baseline. The study groups were managed differently, as made obvious by the cumulative input-output of 2,239 ± 3,695 ml in the WP group vs. 142 ± 3,632 ml in the EVLW group. EVLW decreased significantly, and ventilator-days and ICU days were significantly shorter only in patients from the EVLW group. No clinically significant adverse events occurred as a result of following the EVLW group’s algorithm. Despite several limitations, this study found a lower positive fluid balance, especially in patients with pulmonary edema regardless of cause, associated with a reduced EVLW, less ventilator and ICU days.
The usefulness of EVLW may be obvious in the field of mechanical ventilation and weaning from the respirator. In pigs, the application of 10 cm H2O of PEEP reduced EVLW in a time-dependent manner and maximum protective effect was achieved if it was applied immediately after lung injury production [70]. Colmereno-Ruiz [71] showed in an animal model of ARDS that inappropriate mechanical ventilation (no PEEP, high tidal volume of 12 ml/kg) aggravated lung damage as estimated by EVLW when compared to a protective mode (PEEP 10 cmH2O, tidal volume 6 ml/kg). Zeravik et al. [72] studied patients with ALI and suggested that EVLW may be useful in deciding when to switch from controlled mechanical ventilation to assisted spontaneous breathing mode (EVLW <11 ml/kg). Thus, EVLW may be helpful in guiding fluid management and respirator treatment.
In patients undergoing cardiac surgery, Goepfert et al. [73] studied an algorithm including an EVLW maximum of 10 ml/kg as a limit for fluid treatment. While historical control and goal-directed group (each n=40) had no difference in hard criteria (length of ICU stay or mortality), duration of catecholamine and vasopressor dependence was shorter, and duration of mechanical ventilation and time to achieving status of fit for ICU
discharge was shorter in the study group. EVLW may be of value as an indicator for the prognosis and severity of illness in patients with ALI and ARDS. EVLW-guided therapy has the potential to reduce the duration of mechanical ventilation, ICU length of stay and even reduce mortality in critically ill patients. While systems for the measurement of EVLW are being introduced into the market [74], larger trials are warranted to confirm these findings in the future.
Conclusion
Particularly in patients with ARDS, inflammatory processes in the lungs may increase capillary permeability, causing the accumulation of EVLW which is associated with lung edema and a significant reduction in pulmonary function. EVLW can be safely measured at the bedside by transpulmonary indicator dilution and may be useful for clinical management. As has been shown in clinical and experimental studies, single
transpulmonary thermodilution correlates closely with gravimetry and the transpulmonary double indicator dilution technique. Dynamic changes in EVLW are of obvious clinical value allowing close monitoring of pulmonary edema at the bedside. EVLW has been demonstrated to correlate with the severity of lung damage in sepsis and ARDS and to have prognostic properties. Thus, monitoring of EVLW seems to be a promising tool
in the early goal-directed therapy of critically ill patients, with further clinical studies required to demonstrate the benefit of such a strategy.
Key messages:
The extravascular lung water as a measure of pulmonary edema can be derived accurately by single transpulmonary thermodilution. In particular, in patients with ARDS the extravascular lung water and its changes cannot be estimated by the hydrostatic component alone. The extravascular lung water is of prognostic relevance in critically ill patients and an independent factor for survival. Goal directed treatment strategies including the extravascular lung water have to potential to shorten the length of mechanical ventilation and stay in the ICU. Future studies focusing on improving outcome of critically by an EVLW guided treatment in critically ill patients are required.
References
1. Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967; 2: 319-23.
2. Staub NC, Hyde RW, Crandall E. NHLBI workshop summary: workshop on techniques to evaluate lung alveolar microvascular injury. Am Rev Respir Dis 1990; 141:1071– 7.
3. Sibbald WJ, Warshawski FJ, Short AK, Harris J, Lefcoe MS, Holliday RL. Clinical studies of measuring extravascular lung water by the thermal dye technique in critically ill patients. Chest 1983; 83: 725-31.
4. Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. A prospective study of lung water measurement during patient management in an intensive care unit. Am Rev Respir Dis 1987; 136: 662 – 8.
5. Mitchell JP, Schuller D, Calandrino FS, Schuster DP. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 1992; 145: 990 – 8.
6. Allison RC, Carlile PV Jr, Gray BA. Thermodilution measurement of lung water. Clin Chest Med 1985; 6: 439 – 57
7. Sakka SG, Rühl CC, Pfeiffer UJ, Beale R, McLuckie A, Reinhart K, Meier-Hellmann A. Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intensive Care Med 2000; 26: 180-7.
8. Belda FJ, Aguilar G, Teboul JL, Pestaña D, Redondo FJ, Malbrain M, Luis JC, Ramasco F, Umgelter A, Wendon J, Kirov M, Fernández-Mondéjar E; PICS Investigators Group. Complications related to less-invasive
haemodynamic monitoring. Br J Anaesth 2011; 106: 482-6.
9. Jambrik Z, Monti S, Coppola V, Agricola E, Mottola G, Miniati M, Picano E. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004; 93: 1265-70.
10. Jambrik Z, Gargani L, Adamicza A, Kaszaki J, Varga A, Forster T, Boros M, Picano E. B-lines quantify the lung water content: a lung ultrasound versus lung gravimetry study in acute lung injury. Ultrasound Med Biol 2010; 36: 2004-10.
11. Trezzi M, Torzillo D, Ceriani E, Costantino G, Caruso S, Damavandi PT, Genderini A, Cicardi M, Montano N, Cogliati C. Lung ultrasonography for the assessment of rapid extravascular water variation: evidence from hemodialysis patients. Intern Emerg Med 2011
12. Lichtenstein D. Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med 2012; 6: 155-62.
13. Kuzkov VV, Suborov EV, Kirov MY, Waerhaug K, Mortensen R, Kuklin VN, Nordhus KC, Bjertnaes LJ. Radiographic lung density assessed by computed tomography is associated with extravascular lung water content. Acta Anaesth Scand 2010; 54: 1018-26
14. Hayes CE, Case TA, Aillion DC, et al. Lung water quantification by nuclear magnetic resonance imaging. Science 1982; 216: 1313-5.
15. Wollmer, Rhodes CG. Positron emission tomography in pulmonary edema. J Thorac Imaging 1988; 3:44-50.
16. Nierman DM, Eisen DI, Fein ED, Hannon E, Mechanick JI, Benjamin EJ. Transthoracic bioimpedance can measure extravascular lung water in acute lung injury. Surg Res 1996; 65: 101-8.
17. Sibbald WJ, Short AK, Warshawski FJ, Cunningham DG, Cheung H. Thermal dye measurements of extravascular lung water in critically ill patients. Intravascular Starling forces and extravascular lung water in the adult respiratory distress syndrome. Chest 1985; 87: 585-92.
18. Hill SL, Elings VB, Lewis FR. Changes in lung water and capillary permeability following sepsis and fluid overload. J Surg Res 1980; 28:140-50.
19. Michard F, Zarka V, Alaya S. Better characterization of acute lung injury/ARDS using lung water. Chest 2004; 125: 1166-7.
20. Newman EV, Merrell M, Genecin A, Monge C, Milnor WR, McKeever WP. The dye dilution method for describing the central circulation. An analysis of factors shaping the time-concentration curves. Circulation 1951; 4: 735-46.
21. Neumann P. Extravascular lung water and intrathoracic blood volume: double versus single indicator dilution technique. Intensive Care Med 1999; 25: 216-9.
22. Fernández-Mondéjar E, Rivera-Fernández R, García-Delgado M, Touma A, Machado J, Chavero J.Small increases in extravascular lung water are accurately detected by transpulmonary thermodilution. J Trauma 2005; 59: 1420-3.
23. Hofmann D, Klein M, Wegscheider K, Sakka SG. Extended hemodynamic monitoring using transpulmonary thermodilution Influence of various factors on the accuracy of the estimation of intrathoracic blood volume and extravascular lung water in critically ill patients. Anaesthesist 2005; 54: 319-26.
24. Katzenelson R, Perel A, Berkenstadt H, Preisman S, Kogan S, Sternik L, Segal E. Accuracy of transpulmonary thermodilution versus gravimetric measurement of extravascular lung water. Crit Care Med 2004; 32:1550-4.
25. Craig TR, Duffy MJ, Shyamsundar M, McDowell C, McLaughlin B, Elborn JS, McAuley DF. Extravascular lung water indexed to predicted body weight is a novel predictor of intensive care unit mortality in patients with acute lung injury. Crit Care Med 2010; 38: 114-20.
26. Sakka SG, Klein M, Reinhart K, Meier-Hellmann A. Prognostic value of extravascular lung water in
critically ill patients. Chest 2002; 122: 2080-6.
27. Fernández-Mondéjar E, Rivera-Fernández R, García-Delgado M, Touma A, Machado J, Chavero J. Small increases in extravascular lung water are accurately detected by transpulmonary thermodilution. J Trauma 2005; 59: 1420-3
28. Faybik P, Hetz H, Baker A, Yankovskaya E, Krenn CG, Steltzer H. Iced versus room temperature injectate for assessment of cardiac output, intrathoracic blood volume, and extravascular lung water by single transpulmonary thermodilution. J Crit Care 2004; 19:103-7.
29. Michard F, Schachtrupp A, Toens C. Factors influencing the estimation of extravascular lung water by transpulmonary thermodilution in critically ill patients. Crit Care Med 2005; 33: 1243-7.
30. Rossi P, Wanecek M, Rudehill A, Konrad D, Weitzberg E, Oldner A. Comparison of a single indicator and gravimetric technique for estimation of extravascular lung water in endotoxemic pigs. Crit Care Med 2006; 34:1437-43.
31. Groeneveld AB, Verheij J. Extravascular lung water to blood volume ratios as measures of permeability in sepsis-induced ALI/ARDS. Intensive Care Med 2006; 32:1315-21.
32. Monnet X, Anguel N, Osman D, Hamzaoui O, Richard C, Teboul JL. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med 2007; 33: 448-53.
33. Chew MS, Ihrman L, During J, Bergenzaun L, Ersson A, Undén J, Ryden J, Akerman E, Larsson M. Extravascular lung water index improves the diagnostic accuracy of lung injury in patients with shock. Crit Care 2012; 16: R1
34. Tagami T, Kushimoto S, Yamamoto Y, Atsumi T, Tosa R, Matsuda K, Oyama R, Kawaguchi T, Masuno T, Hirama H, Yokota H. Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care 2010; 14: R162
35. Eichhorn V, Goepfert MS, Eulenburg C, Malbrain ML, Reuter DA. Comparison of values in critically ill patients for global end-diastolic volume and extravascular lung water measured by transcardiopulmonary thermodilution: A metaanalysis of the literature. Med Intensiva 2012 (epub ahead)
36. Oppenheimer L, Elings VB, Lewis FR. Thermal-dye lung water measurements: effects of edema and embolization. J Surg Res 1979; 26: 504-12.
37. Schreiber T, Hüter L, Schwarzkopf K, Schubert H, Preussler N, Bloos F, Gaser E, Karzai W. Lung perfusion affects preload assessment and lung water calculation with the transpulmonary double indicator method. Intensive Care Med 2001; 27: 1814-8.
38. Phillips CR, Smith SM. Predicted body weight-indexed extravascular lung water is elevated in acute respiratory distress syndrome. Crit Care Med 2009; 37: 377-8.
39. Roch A, Michelet P, D'journo B, Brousse D, Blayac D, Lambert D, Auffray JP. Accuracy and limits of transpulmonary dilution methods in estimating extravascular lung water after pneumonectomy. Chest 205; 128: 927-33.
40. Kuzkov VV, Suborov EV, Kirov MY, Kuklin VN, Sobhkhez M, Johnsen S, Waerhaug K, Bjertnaes LJ. Extravascular lung water after pneumonectomy and one-lung ventilation in sheep. Crit Care Med 2007; 35: 1550-9.
41. Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res 1965; 16: 482-8.
42. Fernandez-Mondejar E, Castano-Perez J, Rivera-Fernandez R, et al. Quantification of lung water by transpulmonary thermodilution in normal and edematous lung. J Crit Care 2003; 18: 253-8.
43. Martin GS, Bernard GR. Airway and lung in sepsis. Intensive Care Med 2001; 27:S63-S79.
44. Halperin BD, Feeley TW, Mihm FG, Chiles C, Guthaner DF, Blank NE. Evaluation of the portable chest roentgenogram for quantitating extravascular lung water in critically ill adults. Chest 1985; 88: 649-52.
45. Boussat S, Jacques T, Levy B, et al. Intravascular volume monitoring and extravascular lung water in septic patients with pulmonary edema. Intensive Care Med 2002; 28: 712-8.
46. Baudendistel L, Shields JB, Kaminski DL. Comparison of double indicator thermodilution measurements of extravascular lung water (EVLW) with radiographic estimation of lung water in trauma patients. J Trauma 1982; 22:983-8.
47. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–49.
48. Schuller D, Mitchell JP, Calandrino FS, Schuster DP. Fluid balance during pulmonary edema. Is fluid gain a marker or a cause of poor outcome? Chest 1991; 100: 1068-1075
49. Sakr Y, Vincent JL, Reinhart K, Groeneveld J, Michalopoulos A, Sprung CL, Artigas A, Ranieri VM; Sepsis Occurence in Acutely Ill Patients Investigators. High tidal volume and positive fluid balance are associated with worse outcome in acute lung injury. Chest 2005; 128: 3098-3108.
50. LeTourneau JL, Pinney J, Phillips CR. Extravascular lung water predicts progression to acute lung injury in patients with increased risk. Crit Care Med 2012; 40: 847-54.
51. Aman J, Groeneveld AB, van Nieuw Amerongen GP. Predictors of pulmonary edema formation during fluid loading in the critically ill with presumed hypovolemia. Crit Care Med 2012; 40: 793-9.
52. Phillips CR, Chesnutt MS, Smith SM. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med 2008; 36: 69-73.
53. Kuzkov VV, Kirov MY, Sovershaev MA, Kuklin VN, Suborov EV, Waerhaug K, Bjertnaes LJ. Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury. Crit Care Med 2006; 34: 1647-53.
54. Deeren DH, Dits H, Daelemans R, Malbrain MLNG. Effect of pleural fluid on the measurement of extrasvascular lung water by single transpulmonary thermodilution. Clin Intensive Care 2004; 15: 119-22.
55. Saugel B, Phillip V, Ernesti C, Messer M, Meidert AS, Schmid RM, Huber W. Impact of large-volume thoracentesis on transpulmonary thermodilution-derived extravascular lung water in medical intensive care unit patients. J Crit Care 2012 [Epub ahead of print]
56. Sakka SG, Hanusch T, Thuemer O, Wegscheider K. Influence of veno-venous renal replacement therapy on transpulmonary thermodilution measurements. Anesth Analg 2007; 105: 1079-82.
57. Haller M, Zöllner C, Manert W, Briegel J, Kilger E, Polasek J, Hummel T, Frost H, Peter K. Thermodilution cardiac output may be incorrect in patients with venovenous extracorporeal lung assist. Am J Resp Crit Care Med 1995; 152: 1812-7.
58. Mross M, Sakka SG. Influence of different blood flows through a pumpless lung assist system on transpulmonary thermodilution-derived variables. Intensive Care Med 2010; 36: 369-70.
59. Sakka SG. Influence of an extracorporeal lung assist system on transpulmonary thermodilution-derived variables. Br J Anaesth 2010; 104: 664-5.
60. ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307: 2526-33.
61. Schuster DP. Identifying patients with ARDS: time for a different approach. Intensive Care Med 1997; 23: 1197-1203.
62. Michard F, Fernandez-Mondejar E, Kirov MY, Malbrain M, Tagami T. A new and simple definition for acute lung injury. Crit Care Med 2012; 40: 1004-6.
63. Camporota L, De Neef M, Beale R. Extravascular lung water in acute respiratory distress syndrome: potential clinical value, assumptions and limitations. Crit Care 2012; 16:114.
64. Pino-Sánchez F, Lara-Rosales R, Guerrero-López F, Chamorro-Marín V, Navarrete-Navarro P, Carazo-de la Fuente E, Fernández-Mondéjar E. Influence of extravascular lung water determination in fluid and vasoactive therapy. J Trauma 2009; 67: 1220-4.
65. Boussat S, Jacques T, Levy B, et al. Intravascular volume monitoring and extravascular lung water in septic patients with pulmonary edema. Intensive Care Med 2002; 28: 712-8.
66. Kuntscher MV, Czermak C, Blome-Eberwein S, Dacho A, Germann G. Transcardiopulmonary thermal dye versus single thermodilution methods for the assessment of intrathoracic blood volume and extravascular lung water in major burn resuscitation. J Burn Care Rehabil 2003; 24:142-7.
67. Branski LK, Herndon DN, Byrd JF, Kinsky MP, Lee JO, Fagan SP, Jeschke MG (2011) Transpulmonary thermodilution for hemodynamic measurements in severely burned children. Crit Care 15: R118
68. Hoeft A, Schorn B, Weyland A, Scholz M, Buhre W, Stepanek E, Allen SJ, Sonntag H. Bedside assessment of intravascular volume status in patients undergoing coronary bypass surgery. Anesthesiology 1994; 81: 76-86.
69. Sturm JA. Entwicklung und Bedeutung der Lungenwassermessung in Klinik und Experiment. In: Bergmann H, Gilly H, Steinbereithner K et al. (ed) Beiträge zur Anästhesiologie und Intensivmedizin. Wien, Austria: Verlag Wilhelm Maudrich 1984, 15-39
70. Fernández Mondéjar E, Vazquez Mata G, Cárdenas A, Mansilla A, Cantalejo F, Rivera R. Ventilation with positive end-expiratory pressure reduces extravascular lung water and increases lymphatic flow in hydrostatic pulmonary edema. Crit Care Med. 1996; 24: 1562-7.
71. Colmenero-Ruiz M, Fernandez-Mondejar E, Fernandez-Sacristan MA, Rivera-Fernandez R, Vazquez-Mata G. PEEP and low tidal volume ventilation reduce lung water in porcine pulmonary edema. Am J Respir Crit Care Med 1997; 155: 964-70.
72. Zeravik J, Borg U, Pfeiffer UJ. Efficacy of pressure support ventilation dependent on extravascular lung water. Chest 1990; 97: 1412 – 1419
73. Goepfert MS, Reuter DA, Akyol D, Lamm P, Kilger E, Goetz AE. Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients. Intensive Care Med 2007; 33: 96-103.
74. Bendjelid K, Giraud R, Siegenthaler N, Michard F. Validation of a new transpulmonary thermodilution system to assess global end-diastolic volume and extravascular lung water. Crit Care 2010; 14: R209