Introduction
Because ALI may arise from diverse and heterogeneous clinical insults, monitoring strategies for patients with ALI is similarly heterogeneous as well. In this review I have divided the monitoring strategies for ALI into three distinct phases. The “at risk phase” is the period in which patients are at risk for ALI and interventions may be applied to minimize or eliminate this risk. The “ALI phase” is the period during which ALI has occurred and requires attentive clinical management. The “resolution phase” is the period defined by resolution of ALI and successful discontinuation of mechanical ventilation. I recognize these phases are arbitrary, but they provide a useful framework for discussing the temporal changes in patient condition and monitoring goals in ALI.
Methods of monitoring?
This chapter is not intended as an exhaustive review of medical devices, particularly given the spate of new monitoring technologies that have arrived in the past few years. However, I will briefly describe the most commonly available technologies to facilitate understanding of the options. The first and most traditional form of monitoring is the pulmonary artery catheter (PAC). By placing this catheter into a large central vein it can be flow-directed into the pulmonary artery. At that location it can use thermodilution techniques to measure cardiac output, as well as to measure the saturation of central venous or mixed-venous blood (ScvO2 or SvO2, respectively), as measures of systemic oxygen delivery and consumption (recall that O2extraction = 1-SvO2). Three important advantages of the PAC are its familiarity, the ability to measure pulmonary artery pressures, and ability to detect changes in blood oxygen saturation between the central venous circulation and the pulmonary arterial circulation, as in patients with a left-to-right shunt. Newer versions of the PAC are available that offer continuous cardiac output and SvO2 monitoring. The primary disadvantages of the PAC are its inability to predict fluid responsiveness or to measure left ventricular preload, as with parameters like stroke volume variation (SVV) or pulse pressure variation (PPV). In addition, there are concerns about adverse effects related to PAC use that will be covered later.
The next most common invasive monitoring technology utilizes transpulmonary thermodilution (TPTD). These systems, of which readily available examples include the PiCCO and EV1000 systems, measure cardiac output using the same thermodilution principles except changes in temperature are detected in a systemic artery rather than in the proximal pulmonary artery. The LiDCO system works similarly but uses lithium dilution rather than thermodilution. TPTD systems have the advantage of detecting the thermodilution curve after transit through the pulmonary circuit, which permits measurement of global or specific end-diastolic volumes and extravascular lung water (EVLW). In addition, because the thermal signal is detecting further downstream, it is less affected by variations in heart rate or changes in respirations.
The newest additions to the hemodynamic monitoring field are less invasive or even non-invasive, using technologies such as non-calibrated (formulaic) hemodynamic monitoring devices based upon arterial waveform analysis (such as the Edwards Flotrac/Vigileo system, the Pulsion ProAQT system and the LiDCO rapid system) and electrical impedance tomography, bioimpedance and bioreactance technologies that detect changes in electrical current across the thorax (such as the Cheetah NICOM device). These devices also have potential application for monitoring of ALI patients and they function similarly to the primary invasive and calibrated devices.
At Risk Phase
In this phase before the onset of ALI, we identify patients at risk for ALI and attempt to optimize clinical management to mitigate or eliminate that risk.1 Because ALI results from diverse clinical insults, the management in the pre-ALI phase will appropriately differ between patients. For purposes of this review, because sepsis is the most common cause of ALI, I have selected septic shock as the prototypical “at risk” disease paradigm requiring invasive monitoring and management.2 Although not all conditions will behave in the same way (aspiration, for example), at least some of the conditions and their management will be mirrored by sepsis (e.g. trauma, pancreatitis).
The current paradigm for optimal management of septic shock is defined by early goal-directed therapy (EGDT). This approach is espoused by the international Surviving Sepsis Campaign and by professional societies as the best evidence-based approach to the care of these patients.3 In this context, it is important to recall that application of EGDT in the seminal study documented an 82% reduction in the development of respiratory failure after the delivery of EGDT.4 Presumably a significant proportion of respiratory failure in patients with septic shock represents ALI, thus it is a reasonable assumption that early and appropriate application of EGDT may prevent the development of ALI.
Monitoring of septic shock patients who are at high risk for ALI is accomplished by standard monitoring as demonstrated in the EGDT trial, and by additional measures that are individualized to the patient. The first component of EGDT is the measurement and “normalization” of central venous pressure (CVP). In the original EGDT trial, the first step in sepsis resuscitation was to administer crystalloid or colloid intravenous fluids until the CVP reached 8-12 mm Hg. Subsequent recommendations involving EGDT, particularly in the SSC guidelines, recommend fluid administration until the CVP reaches 8-12 mm Hg in spontaneously breathing patients, or 12-15 mm Hg in patients who are ventilated with positive pressure.3This modification is intended to account for the positive intrathoracic pressure that is transmitted to the measured CVP, thus reducing the effective CVP from the measured value.
There are limitations to keep in mind when using CVP for patient care. A significant initial problem with relying upon CVP as a guide to fluid management in septic shock is that CVP is an unreliable measure of intravascular volume, cardiac preload, cardiac function or responsiveness to a fluid challenge.5,6 Because CVP is used to guide fluid administration in the EGDT algorithm, the inability of CVP to accurately depict preload or fluid responsiveness is a significant limitation. Another concern is that the CVP target for EGDT is arbitrary and not by itself evidence-based. The two concerns may contribute to the observation that septic shock patients who receive EGDT and receive greater resuscitation (higher CVP after EGDT) have lower survival than those with less fluid administration and lower CVP measures.7 Volumetric parameters, such as end-diastolic volume that may be obtained by transpulmonary thermodilution (TPTD) systems or from continuous cardiac output pulmonary artery catheters (PAC) with right-ventricular ejection fraction measurements are superior measures of cardiac preload.6,8 Specifically, end-diastolic volume by TPTD has been shown an accurate estimate of preload in septic shock patients.9
The second major monitoring component for EGDT is measuring the oxygen saturation of central venous blood (ScvO2). For EGDT, ScvO2 is a major target for therapy, for which low values may result in the administration of supplemental oxygen, transfusion of packed red blood cells or continuous intravenous infusion of inotropes. This being the major difference in EGDT resuscitation compared to prior sepsis resuscitation practices, measuring and titrating therapy based upon ScvO2 is an important part of hemodynamic monitoring in this population. An important caveat in our efforts to normalize ScvO2 is that values above 70%, albeit considered normal in the EGDT algorithm, may not reflect adequate tissue oxygenation in patients with the cellular metabolic derangements common to sepsis.10 Two additional parameters that may help to determine treatment in patients with normal ScvO2 are lactate clearance and the difference between central venous and arterial carbon dioxide (A-v[CO2]).11,12 These additional parameters may identify patients with additional resuscitation needs despite achieving an ScvO2 of > 70%.
The primary concerns with ScvO2 monitoring involve logistics, accuracy and interpretation. The first two parts are inter-related, as ScvO2 can be measured either directly and continuously with intravascular fiberoptic catheters, or by aspiration of blood from a thoracic central venous catheter (CVC) and sending the sample for analysis in a blood gas syringe. The former approach is generally considered accurate and is more timely, but at a higher expense and potential need for catheter replacement if a standard CVC was already in place. The latter approach is primarily limited by proximity of the blood gas laboratory to the patient care area, and the ability to receive timely results. These differences also contribute to accuracy, particularly if the catheter system is not calibrated and maintained properly, or if the blood gas samples are not analyzed in a timely manner in a well-run lab.
All clinicians who have cared for patients with septic shock and delivered EGDT recognize the problem of knowing when to declare defeat in our attempts to normalize ScvO2. The question at that point becomes, “what parameters should be used to guide fluids and vasoactive therapies?” In this group of patients, individualization of therapy may be more important than for the EGDT responders, who may represent those within the standard confidence limits of a bell-shaped curve. For those patients who fail to normalize ScvO2, other considerations must be taken to optimize clinical outcomes, as failure of response to EGDT cannot be taken as an ultimately fatal prognosis or a decision to limit further interventions. Rather, additional fluid therapy should be considered, particularly if further responsiveness is documented and fluid optimization can be achieved. However, fluid therapy must be individualized and goal-directed, as over-resuscitation with more positive fluid balance has been associated with development of ALI.13 And alternatively, additional parameters may be appropriate to guide fluid and vasoactive therapies, such as end-diastolic volumes, extravascular lung water (EVLW) and lactate clearance.11
With the advent of rapid signal processing and efficient computing power, calculation of extravascular lung water (EVLW) has become available at the bedside for patient care. The original methods for measuring EVLW requiring double-indicator dilution techniques were cumbersome, time consuming, more expensive, and lacked simple repeatability.14,15 The modern single-dilution systems permit rapid calculation of EVLW as part of normal hemodynamic assessment at the bedside using thermodilution techniques from an indwelling CVC and a thermistor-tipped arterial catheter.16–19 One significant advantage of the modern devices for measuring EVLW is their concomitant calculations of continuous cardiac output, stroke volume, end-diastolic volume and measures of fluid responsiveness such as stroke volume variation (SVV) or pulse pressure variation (PPV). Although the latter measures are less able to characterize fluid responsiveness in ARDS patients receiving pressure-limited low tidal volume ventilation,20,21 the combination of parameters facilitate hemodynamic optimization and fluid resuscitation in patients at risk for ALI.
Clinical studies have consistently demonstrated that EVLW is prognostically relevant in critically ill patients.22,23 The most recent studies have shown its ability in sepsis patients to predict both the development of ALI as well as multiple organ dysfunction syndrome (MODS) – the most common cause of death with sepsis.24–26 As one might expect from the non-ALI studies, EVLW is at least as prognostically relevant specifically for ALI patients.27–29
The pulmonary artery catheter (PAC) has long been used in invasive monitoring of patient with ALI. It’s use over the past 20 years has been chronicled in various countries, showing an inexorable decline in critically ill patients.30,31 Fortunately, this reduction in use is likely to be an appropriate change in practice, particularly given concerns about both PAC safety and efficacy in monitoring critically ill patients.32,33 As mentioned above, the vascular pressures measured by PAC are infrequently of value in critically ill patients, and the advent of new monitoring devices has permitted the calculation of cardiac output more simply and even non-invasively. From an evidence perspective, a single large study using the PAC in patients at risk for ALI failed to demonstrate any clinical benefit.34
Additional basic parameters to consider measuring in the at risk phase of ALI vary from simple variables that we take for granted (e.g. systemic blood pressure) to more complex variables that require complex measurement schemes (e.g. plasma volume or total blood volume). Although there are few if any data substantiating the value of the latter measures, it is this authors opinion that the former measure has clinical value. Because of the unstable nature of a high proportion of ALI patients, invasive monitoring of arterial blood pressure is justified, both for monitoring blood pressure itself as a hemodynamic parameter but also for monitoring the respiratory system with arterial blood gas analysis.
ALI Phase
Once ALI has occurred, invasive hemodynamic monitoring may be utilized for two specific reasons. The first is to continue optimizing the general care of the patient, often more in association with the underlying cause of ALI than with the ALI itself. The second is to optimize the fluid balance considering both ALI and non-ALI conditions, particularly if they represent potential therapeutic conflicts (such as hydrostatic pressure often does for renal perfusion vs. pulmonary edema).35 These two reasons are tightly inter-related, and delivering the right care to ALI patients requires that we consider them together. For example, optimized fluid and vasoactive therapy in patients with sepsis may improve arterial blood oxygenation, while fluid limited approaches to minimize lung edema may adversely affect renal or intestinal perfusion.
Although I will not further discuss arterial pressure measurements specifically in this management phase, ALI patients are wholly appropriate for placement of an arterial catheter. As with patients in the at risk phase, invasive monitoring of blood pressure and measurement of arterial blood gases is critical to proper patient care in patients with established ALI.
As ALI becomes the prominent feature of the patients critical illness, such as when shock resuscitation has been optimized or other organ dysfunction has been mitigated, fluid balance becomes a primary concern.36This is emphasized by the finding that cumulative positive fluid balance is strongly associated with worse clinical outcomes among patients with ALI.13,37 Although flow (cardiac output) and perfusion (SvO2) may be measured with a PAC, the vascular pressures measured by PAC do not correlate well with total body fluid volume or with intravascular volume. This makes the PAC less desirable for monitoring patients with ALI. As mentioned earlier, use of the PAC has been associated with adverse outcomes in critically ill patients,32 and more recent clinical trials in critically ill patients have observed adverse effects on non-pulmonary outcomes (such as renal failure)38 or no benefit accrued to the PAC.34,39 For the subset of ALI patients with right ventricular dysfunction (as many as 25%), measures of right ventricular filling and function may be useful to guide therapy.40
These results together led the ARDS Network to conduct a large efficacy trial applying the PAC to clinical practice in patients with ALI. The Fluid and Catheter Treatment Trial (FACTT) was divided into two components: a comparison of PAC to CVC for general monitoring of ALI patients, and a comparison of standard (“liberal”) fluid therapy to conservative fluid therapy in ALI patients. The overall study enrolled 1,000 ALI patients early in their treatment course, and they were randomized to be managed with either a CVC or a PAC, and also randomized to receive either liberal or conservative fluid therapy.41 In comparing the PAC and CVC, there was no difference in mortality (27.4% vs. 26.3% at 60 days), in the duration of mechanical ventilation (ventilator-free days) or in ICU or hospital length of stay. Furthermore, there were no differences in either management or outcomes of the subset of ALI patients with shock, which was 37% and 32% respectively at baseline and occurred at similar rates during the treatment periods.
In distinction to the catheter comparisons, the fluid therapy component of the FACTT study was groundbreaking. Patients managed with the fluid conservative approach had more days alive and free of mechanical ventilation (14.6 vs. 12.1 ventilator-free days, p<0.001) without any significant adverse events from the conservative management protocol. The fluid conservative treatment group was also numerically but not statistically superior in the mortality analysis (25.5% vs. 28.4% at 60 days, p=0.30) and was observed to have less failure of the central nervous system out to 28 days and more failure of the cardiovascular system in the first seven days. (The differences in cardiovascular failure did not persist at the 28 day time point). Albeit small differences, the fluid conservative group was also favored in the percentage of patients requiring dialysis at 60 days (10% vs. 14%, p=0.06).
The findings of the FACTT study are supported by both prior and subsequent clinical studies. An important seminal study combined a fluid conservative approach with pulmonary artery catheterization and measurements of EVLW to guide therapy.42 In this study, patients who received fluid conservative therapy had greater reductions in EVLW, a reduced duration of mechanical ventilation and a shorter stay in the intensive care unit (ICU). Two other randomized clinical trials compared the combination of continuous infusion diuretics with hyperoncotic colloids to either standard fluid therapy or to diuretics alone in patients with ALI.43,44 In these studies, greater reductions in fluid balance were achieved with the combination of colloids and diuretics, which coincided with improvements in oxygenation. In addition, systemic hemodynamics were improved in patients who received the diuretic/colloid combination, particularly in comparison to diuretic monotherapy. Most recently, a retrospective cohort study showed that the best outcomes for sepsis-induced ALI patients were associated with appropriate goal-directed therapy early in the course (pre-ALI) and conservative fluid therapy later in the course (ALI).45 (See Figure 2).
.jpg)
FIGURE 2.Relationship in sepsis-induced ALI patients between clinical outcomes (the risk of in-hospital death) and the combination of appropriate delivery of early-goal directed therapy along with subsequent liberal or conservative fluid management. Reproduced from Murphy, et al.45
Optimizing fluid balance in ALI patients requires attention to both pulmonary and non-pulmonary effects of any intervention. For this reason, invasive hemodynamic monitoring may help to guide fluid therapy, to minimize hydrostatic pressure while ensuring adequate tissue perfusion in a critically ill patient. Although as yet unproven by clinical trials, the addition of EVLW to the other monitored parameters may further guide fluid therapy. In a study of mixed critically ill patients, knowledge of EVLW changes fluid and vasopressor therapy in the majority of patients.46 Algorithms that incorporate EVLW into patient treatment have been tested in other conditions, such as subarachnoid hemorrhage, peri-operative optimization for cardiac surgery,47,48 and trials are underway to test related algorithms in patients with sepsis and/or ALI. Specific to patients with pulmonary edema, one randomized clinical trial of 48 patients compared protocolized therapy incorporating EVLW to standard management and reported that EVLW-guided management led to greater reductions in EVLW and shorter duration of mechanical ventilation.49 Perhaps most importantly, in the subset of patients with sepsis and ALI, mortality was also lower in protocol-managed patients. As mentioned above, a larger follow-up study comparing PAC-guided therapy to EVLW-guided therapy observed reductions in EVLW, duration of mechanical ventilation and lengths of ICU and hospital stay.42
When measuring EVLW in patients it is important to properly index measured values. EVLW is measured in absolute cubic centimeters (cc) and is traditionally indexed to body weight in kilograms (cc/kg). Because the capacity for pulmonary edema is related to lung size, which can be estimated using height and gender, and because an increasing proportion of people are above their ideal body weight, EVLW should be indexed to ideal or predicted body weight rather than actual body weight. Many studies of EVLW in ALI patients documented a small proportion of patients to have normal quantities of EVLW,27 which confounded interpretation and led to questions about the accuracy of EVLW measures. When EVLW is indexed to predicted or ideal body weight, essentially all ALI patients have increased EVLW.28,50
Studies of interventions in ALI patients have often used measures from invasive hemodynamic monitoring as an outcome of interest. For example, trials of pharmacological interventions that may impact the quantity of pulmonary edema have included EVLW as an outcome of interest. This includes the initial BALTI trial, which found a difference in EVLW in ALI patients treated with intravenous beta-agonists, as well as the subsequent BALTI-2 trial and the HARP trial.51–53 Whether EVLW as a primary outcome of studies in either early resuscitation or later “de-resuscitation” improves outcomes remains to be proven. Studies of patients at-risk for ALI and studies to examine the pathophysiology of ALI have included the pulmonary vascular permeability index (PVPI).27 PVPI is generally calculated as the ratio of EVLW to a measure of intravascular volume, most frequently as the ratio of EVLW to pulmonary blood volume or EVLW to intrathoracic blood volume. Given that PVPI simply represents the ratio of EVLW to hydrostatic pressure, it is intuitive that PVPI is invariably increased in patients with permeability forms of pulmonary edema (i.e. ALI) and lower in patients with cardiogenic edema.54,55
For ALI patients, the basic premise of “de-resuscitation” is to minimize the hydrostatic pressure and total body fluid excess to facilitate healing and recovery of the patient at the most rapid pace. The finding from the earlier cited study demonstrating the optimal outcomes for sepsis-induced ALI patients are associated with initial goal-directed therapy and later fluid conservative therapy,45 has been confirmed in another study showing that elevations of CVP and positive fluid balance are associated with worse outcomes in septic shock.56 Furthermore, a growing body of literature is emerging that volume overload has adverse effects not only on the pulmonary system, but even on the kidneys, with acute kidney injury outcomes being worse with fluid overload.57 Given this constellation of findings, a fluid conservative approach for patients who have stabilized, and particularly those with ALI, is recommended. The combination of preload measures and EVLW that are available from invasive hemodynamic monitoring devices should produce better clinical fluid management and superior clinical outcomes. Although the optimal algorithm remains to be defined, a general schematic approach is in Figure 3. In essence, the priority order of parameters shifts from the preload, flow and perfusion measures in the early at-risk phase to the volumetric and safety targets in the ALI and resolution phases. While some measure of early goal-directed and later conservative fluid therapy might be achieved without monitoring, it seems unlikely that the same magnitude and impact of either will be achieved, particularly with the same safety profile.
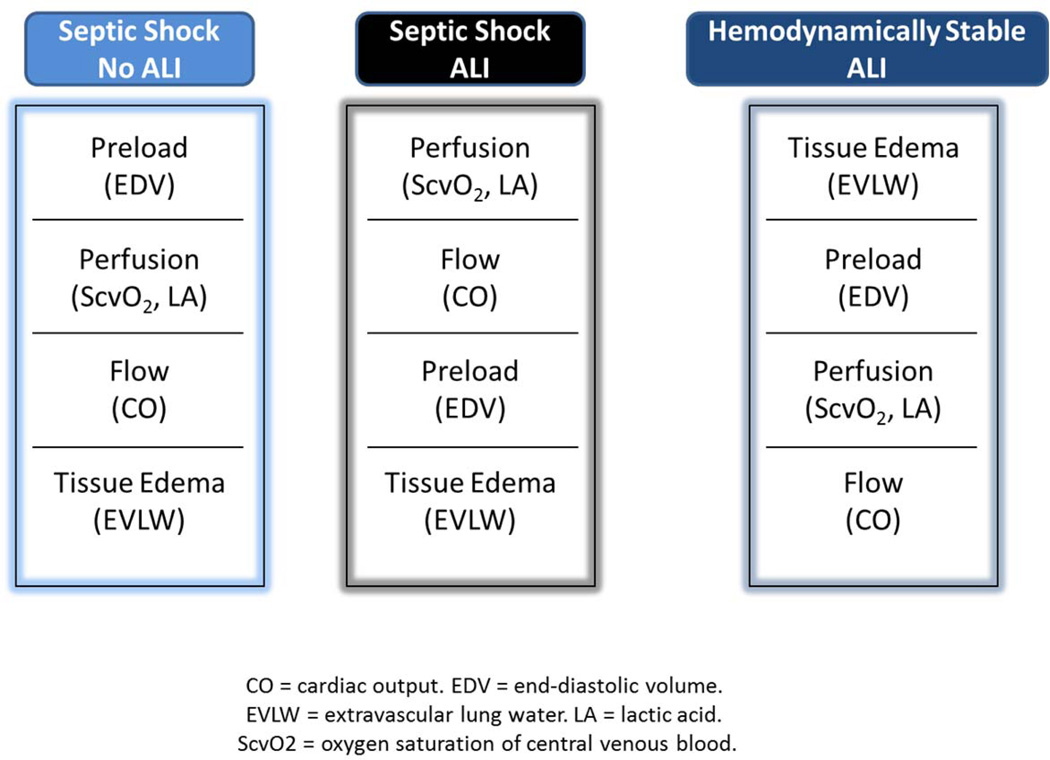
FIGURE 3. Theoretical depiction of how monitoring priorities may change based upon the condition and temporal phase of a prototypical patient with septic shock and acute lung injury.
ALI Resolution Phase
For the purposes of this review, the resolution of ALI is defined by discontinuation of mechanical ventilation. For patients who have successfully resolved ALI and are no longer requiring mechanical ventilation, invasive hemodynamic monitoring is unlikely to be of further benefit. The exception to this is patients with ongoing management for other organ dysfunction or ongoing hemodynamic concerns. This exception aside, removal of invasive devices for hemodynamic monitoring is appropriate, as is acceleration of any other interventions to facilitate patient recovery and acclimatization back towards functional independence.
In cases where ALI resolves but the requirement for mechanical ventilation persists, monitoring may be useful to guide weaning from mechanical ventilation. In this case, invasive hemodynamics, lung water and echocardiography have all been described to have clinical utility. Because fluid removal is one of the more frequent impediments to successful ventilator weaning, monitoring may be useful to guide safe and appropriate de-resuscitation in these patients as well. In addition, monitoring for cardiogenic complications of ventilator weaning, such as ischemic pulmonary edema, may also be facilitated by invasive hemodynamic monitoring during ventilator weaning trials.58
Conclusions
Invasive hemodynamic monitoring has specific roles in each phase of therapy for patients with ALI: pre-ALI, peri-ALI and post-ALI. The primary goals are to optimize fluid resuscitation in order to prevent organ dysfunction, including ALI, and if ALI occurs to additional optimize fluid balance vis-à-vis the lung. By judicious application of invasive hemodynamic monitoring, particularly in its more modern iterations, clinicians can optimize the ebb and flow phases common to critically ill patients. This is vitally important given our current and growing understanding of the relationship between fluid balance and important clinical outcomes, including ALI, AKI, multiple organ dysfunction syndrome, and mortality.
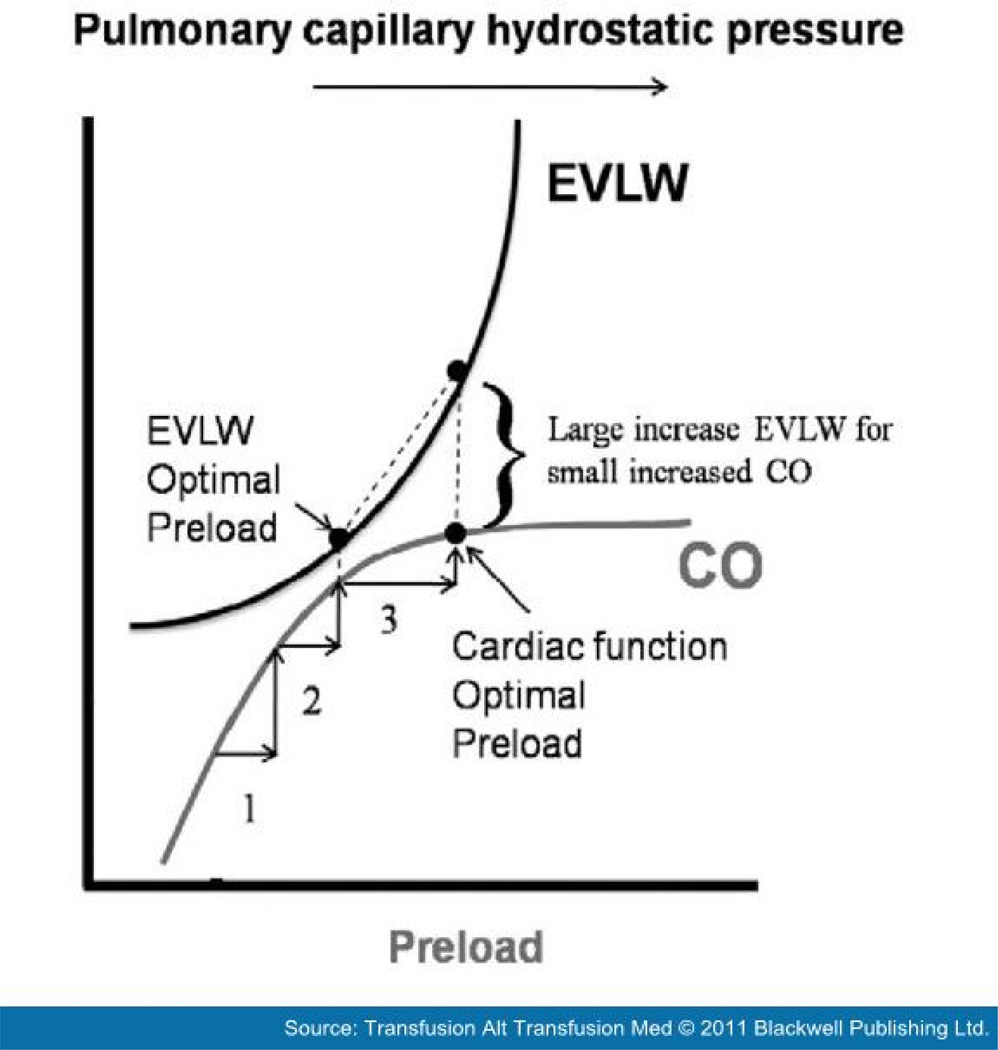
FIGURE 1. The effect of increasing hydrostatic pressure on the potentially competing and related physiological outcomes of cardiac output (CO) and pulmonary edema (extravascular lung water, EVLW). Note that increasing hydrostatic pressure may differentially affect CO and EVLW, depending on the shape of the two curves. Considering these two measures together allows for more precise clinical management and determination of an optimal preload for CO, EVLW or both. Reproduced from: Marik PE. Hemodynamic parameters to guide fluid therapy. Transfusion Alter Transfusion Med 2010; 11(3): 102–112.
Acknowledgments
Supported by the National Institutes of Health (P50 AA-013757, R21 HL-110044, U54 RR-024380) and the Food and Drug Administration (R01 FD-003440).
Reference List