Purpose of review
To discuss the role of the invasive monitoring techniques pulmonary artery catheter (PAC) and transpulmonary thermodilution (TPD) for cardiopulmonary monitoring in the critically ill patient.
Recent findings
Characterization of the nature of hemodynamic alterations and hemodynamic optimization can be achieved both with PAC and TPD. Some recent trials suggest that volumetric measurements may be preferred in conditions with preserved left ventricular systolic function, whereas pressure measurements should be preferred in patients with altered left ventricular systolic function. Extravascular lung water is strongly associated with outcome and may be used to reflect the impact of fluid management strategies. The time response of this measurement needs still to be better defined.
Summary
This review highlights that PAC and TPD have an important role in cardiopulmonary monitoring of critically ill patients. Both techniques can be used efficiently to diagnose the nature of circulatory or respiratory failure and to monitor the effects of therapies. The choice of the technique should be guided by the patient’s condition and the need for additional measurements rather than based on physician’s preferences.
Keywords
cardiac output, extravascular lung water, filling pressures, pulmonary artery catheter, transpulmonary thermodilution
INTRODUCTION
Circulatory failure, or shock, is a life-threatening condition that can arise from multiple disease processes. Shock is associated with mortality rates
around 50% [1,2], but these are markedly influenced by the disease process leading to shock. It is associated with tissue hypoperfusion and cellular dysfunction, leading to organ dysfunction. It requires prompt therapy, aiming at not only controlling its source but also supporting the hemodynamics. The place of hemodynamic monitoring is still debated, even though frequently used. Hemodynamic monitoring helps to identify the type of shock and guiding therapeutic interventions. Invasive techniques such as pulmonary artery catheter (PAC) and transpulmonary thermodilution (TPD) provide important information in these severely ill patients. Another important indication for hemodynamic evaluation with invasive techniques is the cardiopulmonary evaluation of the patient with respiratory failure. In this article, we will review the recent evidence discussing the use of invasive hemodynamic techniques in the cardiopulmonary evaluation of the critically ill patient with circulatory or respiratory failure.
WHAT ARE MEASURING INVASIVE HEMODYNAMIC DEVICES?
The PAC measures cardiac output (CO), pulmonary artery pressure (PAP), pulmonary artery occluded pressure (PAOP) and right atrial pressure (RAP). TPD measures CO, global end-diastolic volume (GEDV), global ejection fraction (GEF), extravascular lung water (EVLW) and pulmonary vascular permeability (PVP). Two techniques are available for TPD: the PiCCO system (Pulsion, Munich, Germany) and the EV1000 (Edwards Lifesciences, Irvine, California, USA). The formulas used by the two devices to compute CO are quite similar, but markedly differ for the computation of derived variables.
Measurements of CO by thermodilution and by the PiCCO system have been validated for a long time. In the pediatric setting, fewer data are available. In a pediatric model of hemorrhagic shock, PAC and TPD similarly tracked the changes in CO during bleeding and resuscitation [3]. In adults also, some technical information, important for routine and investigational use, was published recently. The number of injections required for calibration and derived measurements were evaluated in 91 hemodynamically stable patients [4]. At least three injections should be performed, which allows precision to drop below 10% for CO as well as GEDV and EVLW. Hypothermia may affect this reliability, as signal-to-noise ratio may decrease. In 88 postcardiac arrest patients, the least significant change for CO, GEDV, EVLW and PVP were 7.8, 8.5, 7.8 and 12.1%, respectively, without differences between hypothermic and nonhypothermic conditions [5]. Two other trials demonstrated that continuous renal replacement
therapy does not impact the CO measurements by TPD [6,7]. Finally, a trial in 30 patients undergoing elective mitral valve repair showed that
mitral regurgitation has minimal impact on the agreement between the CO obtained by PAC or by TPD [8].
The EV1000 system has been more recently introduced. It has been initially validated in experimental conditions [9]. In pigs, GEDV and EVLW
were adequately measured with EV1000 and PiCCO systems [close correlation (r2=0.79 and 0.97), minimal bias (11 and 5 ml) and limited percentage errors (14 and 15%)]. Validation of the system was also reported in the clinical setting. In a prospective, multicenter, observational study, the two systems were compared in 72 critically ill patients [10]. During a 72-h observation period, 443 sets of thermodilution measurements were obtained. There was an excellent correlation between the two systems for CO, GEDV and EVLW (r2=0.981, 0.926 and 0.971, respectively) and minimal bias, resulting in a low percentage error (9.7, 11.5 and 12.2%). Changes in CO, GEDV and EVLW were tracked with a high concordance between the two systems. On the basis of these two studies, the two systems seem to perform similarly.
HOW TO DIFFERENTIATE THE TYPE OF SHOCK?
There are four types of shock: hypovolemic, cardiogenic, obstructive and distributive [11]. Whereas distributive shock is usually characterized by high CO and vasodilation, the other types of shock are associated with low CO and vasoconstriction. In hypovolemic shock, pressures and volumes are typically low, whereas these are increased in cardiogenic shock. Obstructive shock is associated with increased PAP and dilated right-sided cavities, compressing left heart cavities. Tamponade, usually comprised into cardiogenic shock, is associated with compression of all cavities, and thus elevated pressures but small volumes.
Diagnosis with PAC is relatively easy (Fig. 1). Distributive shock is usually characterized by high CO and mixed-venous O2 saturation (SvO2), with
filling pressure in the normal to low range. In hypovolemic shock, CO, SvO2 and all pressures are typically low. In cardiogenic shock, CO and SvO2
are low, whereas PAOP and RAP are high. Obstructive shock is associated with low CO and SvO2, and increased PAP and RAP, with a RAP which exceeds PAOP. Tamponade is associated with pulsus paradoxus, low CO and SvO2, together with equally elevated RAP, PAOP and diastolic PAP.
.jpg)
FIGURE 1. Characterization of the hemodynamic alterations in patients with circulatory failure using PAC. This algorithm allows to identify the different types of shock and to characterize the main process implicated in circulatory failure.
LV, left ventricle; PAC, pulmonary artery catheter; PAOP, pulmonary artery occluded pressure; PAP, pulmonary artery pressure; RAP, right atrial pressure; RV, right ventricle.
With TPD, the diagnosis is also easy except for obstructive shock (Fig. 2). Distributive shock is usually characterized by high CO and central venous
O2 saturation (ScvO2), with GEDV in the normal to low range. In hypovolemic shock, CO, ScvO2 and GEDV are typically low. In cardiogenic shock, CO and ScvO2 are low, whereas GEDV is high. Obstructive shock is associated with low CO and ScvO2, and increased GEDV. Without echocardiographic assessment, this shock cannot be distinguished from cardiogenic shock. Tamponade is associated with pulsus paradoxus, low CO, low GEDV and ScvO2.
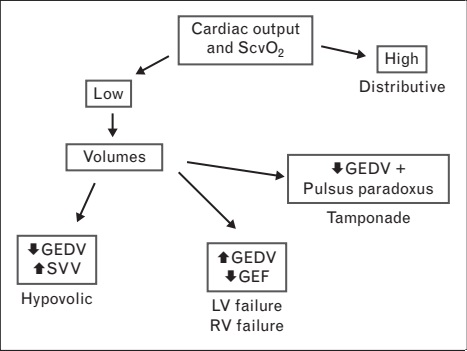
FIGURE 2. Characterization of the hemodynamic alterations in patients with circulatory failure using TPD. This algorithm allows to identify the different types of shock and to characterize the main process implicated in circulatory failure.
GEDV, global end-diastolic volume; LV, left ventricle; RV, right ventricle; SVV, stroke volume variation; TPD, transpulmonary thermodilution.
There is often an overlap between these. Myocardial depression is often observed in distributive shock, hypovolemia may occur in cardiogenic shock. Accordingly, hemodynamic support of circulatory shock should not be focused on one single intervention, but rather intervene on the different alterations. Priority for therapy should be given to the most relevant alteration.
It is now recognized that echocardiographic evaluation should be used in most cases [12]. Nevertheless, invasive techniques keep their diagnostic
interest, especially when changes might have occurred since the initial evaluation. It is possible to categorize the patients into the different shock
categories based either on measurements of CO and pressures with PAC (Fig. 1) or on CO and volumes with TPD (Fig. 2). This has been recently confirmed against echocardiography. In trauma patients, PAC adequately identified patients with moderate-to-severe myocardial dysfunction [13]. In another trial involving 29 trauma patients, CO from PAC and echocardiography were significantly related and agreed with moderate strength [14].
In order to define target values of volumetric measurements for therapies, it is important to determine the values of these variables in the target populations. The proposed normal values for GEDV (680–800 ml/m2) and EVLW (3–7 ml/kg) are based on measurements in critically ill patients without major hemodynamic or respiratory problems. These values may fail to represent values observed in more severely ill, but nevertheless hemodynamically stable, patients. The usual values observed in high-risk surgical and septic patients were reported in a meta-analysis included 64 studies measuring GEDV and EVLW in 1925 patients [15]. GEDV was higher in septic than in high-risk surgical patients [788 (762–816) vs. 694 ml/m2 (678–711), P < 0.001]. EVLW was also higher in septic than in surgical patients [11.0 (9.1–13.0) vs. 7.2 ml/kg (6.9–7.6), P=0.001]. These data suggest that different therapeutic targets should be used in high-risk surgical patients and in septic patients.
USE OF INVASIVE HEMODYNAMIC TECHNIQUES IN GUIDING THERAPY IN SHOCK
Optimization of fluid therapy is a topic of intense research. Cardiac filling volumes and pressures are often used to guide fluid administration. The
relative value of cardiac filling volume and pressures for predicting fluid responsiveness was evaluated in 32 patients after cardiovascular surgery [16].
Regardless of the ejection fraction, baseline CVP was lower in responding events. PAOP was more useful than GEDV for predicting fluid responsiveness when ejection fraction was low, but when ejection fraction is near normal, GEDV is more useful than PAOP. Similar results were observed in 40 patients with complex valvular surgery [17]. Goal-directed therapy was applied either by PAC or by TPD. The TPD group received more fluids, which was associated with a decrease in the duration of mechanical ventilation by 5.2 h compared with the PAC group (P < 0.05). Unfortunately, the hemodynamic goals were not identical, as CO was targeted with PAC and oxygen delivery (DO2) with TPD, so that a higher
DO2 was reached in the TPD group.
In patients with severe sepsis, PAC can also be used for goal-directed therapy. In a before/after study including 2 70 patients, the introduction
of a PAC-based protocol was associated with a significantly higher positive fluid balance in comparison with controls at day 1 (6 vs. 4 l, P < 0.001), but a lower cumulative fluid balance after 7 days (9 vs. 13 l, P=0.001), whereas maximum norepinephrine dose was significantly higher in the PAC group [18]. More importantly, PAC-based protocol was associated with a significant reduction in ventilator and ICU days. A very important randomized trial evaluated PAC and TPD for the management of 120 critically ill patients in shock, including 72 patients with septic and 48 with nonseptic shock [19]. In both groups, hemodynamic management was guided by the algorithms for 72 h after enrollment. In the TPD group, the upper limits for fluid resuscitation were EVLW less than 10 ml/kg and GEDV less than 850 ml/m2. In the PAC group, a PAOP less than 18–20 mmHg was used. Primary outcomes were ventilator-free days and length of stay in the intensive care unit and the hospital. Overall, ventilator free days, length of stay, organ failures and 28-day mortality were similar in both the monitoring groups. Interestingly, in the nonseptic subgroup, TPD was associated with more days on mechanical ventilation and longer intensive care unit and hospital length of stay than PAC (P=0.001). This was not observed in the septic shock subgroup. In both conditions, TPD (vs. PAC) was associated with a more positive fluid balance at 24 h. The main conclusion of this trial is that indeed globally hemodynamic management guided by TPD and PAC yield the same outcomes. The improved outcomes by PAC in nonseptic group may be related to the higher incidence of cardiac dysfunction in this group.
Indeed, when cardiac function is impaired, pressures better predict the response to fluids than volumes [16]. Of course, one cannot rule out that the resuscitation protocol was not optimal in the TPD group. These variables may help to guide therapy. In 37 patients submitted to thoracic surgery requiring one-lung ventilation, a protocol using a goal directed fluid therapy based on stroke volume variation (SVV) did not increase EVLW [20]. Another word of caution should be mentioned for patients with severe burn injury. Thirty adult burned patients (25–60% BSA) received fluid resuscitation either guided by the PiCCO or were resuscitated using Parkland formula associated with traditional monitoring parameters [21]. Fluid administration in the initial 72 h after burn injury was significantly higher in the study group. Nevertheless, CO and derived variables were lower, whereas tissue edema was worse. This implies that either the resuscitative algorithm was not optimal or that other factors other than fluid responsiveness play a role in these patients. This study indirectly emphasizes on the importance of timing in fluid resuscitation [22].
USE OF INVASIVE HEMODYNAMIC TECHNIQUES IN RESPIRATORY FAILURE
Both PAC and TPD can be used to discriminate between hydrostatic and nonhydrostatic causes of pulmonary edema. A PAOP greater than 18 mmHg suggests hydrostatic pulmonary edema. With TPD, EVLW of at least 10ml/kg indicates edema, but not the origin of the edema. However, the pulmonary vascular permeability index, PVP, representing the ratio between EVLW and GEDV, is indicative of nonhydrostatic cause when elevated (above 2.5–3.0).
This was nicely illustrated in a multi-institutional, observational study including 266 patients with a PaO2/FiO2 ratio of 300 mmHg or less and bilateral infiltration on chest radiography [23]. Patients were divided into the following three categories of respiratory insufficiency: acute lung
injury (ALI)/acute respiratory distress syndrome (ARDS) (n=207), cardiogenic edema (n=26) and pleural effusion with atelectasis (n=33). EVLW
was greater in ALI/ARDS and cardiogenic edema patients than in those with pleural effusion with atelectasis (18.5±6.8, 14.4±4.0 and 8.3±2.1, respectively; P < 0.01). PVP was higher in ALI/ARDS patients than in those with cardiogenic edema or pleural effusion with atelectasis (3.2±1.4, 2.0±0.8, 1.6±0.5; P < 0.01). EVLW correlated with PVP, but not PaO2/FiO2. A PVP value of 2.6–2.85 diagnosed ARDS (specificity, 0.90–0.95) and a value less than 1.7 ruled out ARDS (specificity, 0.95). Finally, it seems that EVLW can be used in septic patients to identify patients at risk to develop ARDS 2–3 days later [24].
Several trials investigated the impact of fluids on EVLW. An old study showed that EVLW does not increase acutely after fluid administration [25]. In 17 consecutive postoperative, mechanically ventilated patients presenting with circulatory failure, fluids were administered according to SVV measurements [26]. During the first 12 h after fluid loading, CO increased but EVLW did not increase. No correlation was found between the amount of fluids administered and the changes in EVLW. This implies that either the protocol used was able to limit the increase in hydrostatic pressures or that EVLW measurements are not sensitive enough or that a longer delay should be observed. Another team found more encouraging results on the dynamic aspect of EVLW [27]. In 62 patients with sepsis, fluid administration resulted in an increase in EVLW by at least 10% in 27 patients (44%). These patients had similar hemodynamic variables compared to others, but were characterized by lower albumin levels. Of note, this was not detected by PVP at baseline. In another trial including 63 critically ill patients, an increase in EVLW greater than 10% was observed
in patients on the plateau of the Starling relationship but was not related to baseline PVP, PAOP and albumin levels [28].
On the other hand, fluid retrieval does not rapidly impact EVLW. Even though significant, changes in EVLW during fluid retrieval were not very large. In nine patients with acute renal failure and hydrostatic or nonhydrostatic pulmonary edema, GEDV and EVLW minimally decreased after
ultrafiltration of 3.6 l [29]. However, this effect may take time to be detected. In a case–control series, matching patients receiving a strategy of high PEEP, albumin and fluid removal, EVLW decreased more over 1 week in the interventional arm than in historical control [30]. This was associated with a decrease in days on mechanical ventilation and in ICU length of stay. These promising results were nevertheless limited by the nature of the control group and should be confirmed in prospective trials.
Several trials evaluated the prognostic value of EVLW. In 200 patients with ARDS, the maximum value of EVLW and maximum value of PVP were
significantly higher in nonsurvivors than in survivors at day 28 [24±10 vs. 19±7 ml/kg, P < 0.001, and 4.4 (3.3–6.1) vs. 3.5 (2.8–4.4), P=0.001,
respectively] [31]. These factors remained associated with outcome in multivariate analyses, in addition to other factors such as positive end-expiratory pressure level, cumulative fluid balance and PaO2/FiO2 ratio. This suggests that not only the consequences (impairment of oxygenation), but also the mechanisms and its severity (endothelial dysfunction and capillary leak as reflected by permeability index) are important for the outcome in ARDS. These observations are however limited by the fact that the worst and mean values were evaluated, which are affected by the poor evolution of the patient. The evolution of EVLW was evaluated in 123 critically ill patients, in whom achievement of a negative fluid balance was attempted [32]. Decreases in EVLW by more than 2 ml/kg were more frequently observed in patients achieving a negative fluid balance. These patients also had more ventilator-free days during the first week. Even though the nature of the trial does not allow to define a causal mechanism, these results are in line with the FACTT (Fluid and Catheter Treatment Trial) that showed that fluid-restrictive strategy in ARDS patients increased mechanical ventilation-free days [33].
The relationship between EVLW and outcome was also evaluated in 55 patients with septic shock [34]. At day 1, EVLW and PVP did not differ between survivors and nonsurvivors. However, EVLW and PVP decreased in the survivors, but remained elevated in the nonsurvivors so that
these differed at day 3. EVLW at day 3 remained associated with outcome in multiple logistic regression analysis. The authors also nicely showed
that indexing EVLW for actual or predicted body weight did not affect the results.
CONCLUSION
Both PAC and TPD have an important place in cardiopulmonary monitoring of the critically ill patient. Both techniques can be used efficiently to diagnose the nature of circulatory or respiratory failure, and to monitor the effects of therapies. Each of these techniques has limitations, and the choice of the technique should be guided by the patient’s condition (i.e. pulmonary hypertension, left ventricular systolic and diastolic function, etc.) and by the interest of specific measurements (such as EVLW in given conditions) rather than based on unit preferences.
Acknowledgements
None.
Conflicts of interest
Daniel De Backer received honoraria for lectures from Edwards LifeSciences, Pulsion, Gambro Hospal, Eli Lilly, AM Pharma, Sangart and grant or devices for studies from Edwards LifeSciences, Pulsion, LiDCO, Vygon, Cheetah Medical, Imacor, Gambro Hospal, Eli Lilly and CMA microdialysis.
David Fagnoul and Antoine Herpain have no conflicts of interest to declare.
REFERENCES AND RECOMMENDED READING
1. De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362:779–789.
2. Awad HH, Anderson FA Jr, Gore JM, et al. Cardiogenic shock complicating acute coronary syndromes: insights from the Global Registry of Acute Coronary Events. Am Heart J 2012; 163:963–971.
3. Ballestero Y, Urbano J, Lopez-Herce J, et al. Pulmonary arterial thermodilution, femoral arterial thermodilution and bioreactance cardiac output monitoring in a pediatric hemorrhagic hypovolemic shock model. Resuscitation 2012; 83:125–129.
4. Monnet X, Persichini R, Ktari M, et al. Precision of the transpulmonary thermodilution measurements. Crit Care 2011; 15:R204.
5. Tagami T, Kushimoto S, Tosa R, et al. The precision of PiCCO(R) measurements in hypothermic postcardiac arrest patients. Anaesthesia 2012; 67:236–243.
6. Heise D, Faulstich M, Morer O, et al. Influence of continuous renal replacement therapy on cardiac output measurement using thermodilution techniques. Minerva Anestesiol 2012; 78:315–321.
7. Dufour N, Delville M, Teboul JL, et al. Transpulmonary thermodilution measurements are not affected by continuous veno-venous hemofiltration at high blood pump flow. Intensive Care Med 2012; 38:1162–1168.
8. Staier K, Wilhelm M, Wiesenack C, et al. Pulmonary artery vs. transpulmonary thermodilution for the assessment of cardiac output in mitral regurgitation: a prospective observational study. Eur J Anaesthesiol 2012; 29:431–437.
9. Bendjelid K, Giraud R, Siegenthaler N, et al. Validation of a new transpulmonary thermodilution system to assess global end-diastolic volume and
extravascular lung water. Crit Care 2010; 14:R209.
10. Kiefer N, Hofer CK, Marx G, et al. Clinical validation of a new thermodilution system for the assessment of cardiac output and volumetric parameters. Crit Care 2012; 16:R98.
11. Weil MH, Shubin H. Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 1971; 23:13–23.
12. Vincent JL, Rhodes A, Perel A, et al. Clinical review: update on hemodynamic monitoring – a consensus of 16. Crit Care 2011; 15:229.
13. Murthi SB, Hess JR, Hess A, et al. Focused rapid echocardiographic evaluation versus vascular catheter-based assessment of cardiac output and function in critically ill trauma patients. J Trauma Acute Care Surg 2012; 72:1158–1164.
14. Tchorz KM, Chandra MS, Markert RJ, et al. Comparison of hemodynamic measurements from invasive and noninvasive monitoring during early resuscitation. J Trauma Acute Care Surg 2012; 72:852–860.
15. Eichhorn V, Goepfert MS, Eulenburg C, et al. Comparison of values in critically ill patients for global end-diastolic volume and extravascular lung water measured by transcardiopulmonary thermodilution: a meta-analysis of the literature. Med Intensiva 2012; 36:467–474.
16. Trof RJ, Danad I, Reilingh MW, et al. Cardiac filling volumes versus pressures for predicting fluid responsiveness after cardiovascular surgery: the role of systolic cardiac function. Crit Care 2011; 15:R73.
17. Lenkin AI, Kirov MY, Kuzkov VV, et al. Comparison of goal-directed hemodynamic optimization using pulmonary artery catheter and transpulmonary thermodilution in combined valve repair: a randomized clinical trial. Crit Care Res Pract 2012; 2012:821218.
18. Bethlehem C, Groenwold FM, Buter H, et al. The impact of a pulmonary-arterycatheter-based protocol on fluid and catecholamine administration in early sepsis. Crit Care Res Pract 2012; 2012:161879.
19. Trof RJ, Beishuizen A, Cornet AD, et al. Volume-limited versus pressurelimited hemodynamic management in septic and nonseptic shock. Crit Care Med 2012; 40:1177–1185.
20. Haas S, Eichhorn V, Hasbach T, et al. Goal-directed fluid therapy using stroke volume variation does not result in pulmonary fluid overload in thoracic surgery requiring one-lung ventilation. Crit Care Res Pract 2012; 2012:687018.
21. Aboelatta Y, Abdelsalam A. Volume overload of fluid resuscitation in acutely burned patients using transpulmonary thermodilution technique. J Burn Care Res 2012. [Epub ahead of print] doi: 10.1097/BCR.0b013e3182642b32
22. Correa TD, Vuda M, Blaser AR, et al. Effect of treatment delay on disease severity and need for resuscitation in porcine fecal peritonitis. Crit Care Med 2012; 40:2841–2849.
23. Kushimoto S, Taira Y, Kitazawa Y, et al. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care 2012; 16:R232.
24. LeTourneau JL, Pinney J, Phillips CR. Extravascular lung water predicts progression to acute lung injury in patients with increased risk. Crit Care
Med 2012; 40:847–854.
25. Matejovic M, Krouzecky A, Rokyta R Jr, et al. Fluid challenge in patients at risk for fluid loading-induced pulmonary edema. Acta Anaesthesiol Scand 2004; 48:69–73.
26. Ferrando C, Aguilar G, Belda FJ. Extravascular lung water does not increase in hypovolemic patients after a fluid-loading protocol guided by the stroke volume variation. Crit Care Res Pract 2012; 2012:437659.
27. Zhang Z, Lu B, Ni H, et al. Prediction of pulmonary edema by plasma protein levels in patients with sepsis. J Crit Care 2012; 27:623–629.
28. Aman J, Groeneveld AB, Nieuw Amerongen GP. Predictors of pulmonary edema formation during fluid loading in the critically ill with presumed
hypovolemia. Crit Care Med 2012; 40:793–799.
29. De Laet I, Deeren D, Schoonheydt K, et al. Renal replacement therapy with net fluid removal lowers intra-abdominal pressure and volumetric indices in critically ill patients. Ann Intensive Care 2012; 2 (Suppl. 1):S20.
30. Cordemans C, De Laet I, Van Regenmortel N, et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive
Care 2012; 2 (Suppl. 1):S15.
31. Jozwiak M, Silva S, Persichini R, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress
syndrome. Crit Care Med 2013; 41:472–480.
32. Cordemans C, De Laet I, Van Regenmortel N, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care 2012; 2 (Suppl. 1):S1.
33. Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluidmanagement strategies in acute lung injury. N Engl J Med 2006; 354:2564–2575.
34. Mallat J, Pepy F, Lemyze M, et al. Extravascular lung water indexed or not to predicted body weight is a predictor of mortality in septic shock patients. J Crit Care 2012; 27:376–383.