Abstract
The Surviving Sepsis Campaign guidelines for the management of severe sepsis and septic shock recommend that the initial hemodynamic resuscitation be done according to the protocol used by Rivers and colleagues in their well-known early goal-directed therapy (EGDT) study. However, it may well be that their patients were much sicker on admission than many other septic patients. Compared with other populations of septic patients, the patients of Rivers and colleagues had a higher incidence of severe comorbidities, a more severe hemodynamic status on admission (excessively low central venous oxygen saturation [ScvO2], low central venous pressure [CVP], and high lactate), and higher mortality rates. Therefore, it may well be that these patients arrived to the hospital in late untreated hypovolemic sepsis, which may have been due, in part at least, to low socioeconomic status and reduced access to health care. The EGDT protocol uses target values for CVP and ScvO2 to guide hemodynamic management. However, filling pressures do not reliably predict the response to fluid administration, while the ScvO2 of septic patients is characteristically high due to decreased oxygen extraction. For all these reasons, it seems that the hemodynamic component of the Surviving Sepsis Campaign guidelines cannot be applied to all septic patients, particularly those who develop sepsis during their hospital stay.
Background
The early institution of goal-directed therapy has always been perceived as a key factor for the successful management of critically ill patients. In trauma patients, for example, the early detection of occult hypoperfusion and its correction using goal-directed therapy have been shown to reduce both mortality and morbidity [1]. Nevertheless, the 2001 study by Rivers and colleagues [2] (referred to hereafter as 'the Rivers study') was the first to show that the institution of early goal-directed therapy (EGDT) upon admission to the emergency department (ED) can significantly reduce mortality of patients in severe sepsis or septic shock. The results of the Rivers study are unique because, due to the complexity of hemodynamics in sepsis, the goals of therapy are much more difficult to define with certainty than in other forms of shock [3]. A recent systematic literature review has indeed found a lack of agreement on hemodynamic goals for management of patients with sepsis, proposing that this lack of consistency may contribute to heterogeneity in treatment effects for clinical trials of novel sepsis therapies [4]. Although the challenge of overcoming sepsis has previously prompted the production of practice parameters for hemodynamic support of adult septic patients [3], no evidence has been produced, prior to the Rivers study, that adherence to any such treatment guidelines can improve the dismal prognosis of severe sepsis and septic shock.
Following the Rivers study, critical care and infectious disease experts representing 11 international organizations developed management guidelines for severe sepsis and septic shock under the auspices of the Surviving Sepsis Campaign (SSC) [5]. These guidelines have received worldwide acclaim for being 'a noble, well-intentioned approach to transfer knowledge gained from research into practice at the bedside' [6]. The SSC guidelines were adopted by many medical centers worldwide, a process that is still ongoing and that has led to numerous reports of improved survival [7]. The uncontested success of these guidelines has led to their inclusion in mainstream reviews on the management of sepsis [8] and made opinion leaders recommend that they be adopted by the complete health care network involved in the management of patients with severe sepsis [9].
However, in addition to the recommendations for the initial hemodynamic resuscitation of the septic patient which are based on the Rivers protocol (Table (Table1),1), the SSC guidelines include many other aspects of care, including the early use of antibiotics, tight glucose control, steroids, recombinant human-activated protein C, and many more. In the most recent edition of these guidelines [10], the number of recommendations increased to 85 from the original 52 that appeared in the 2004 edition [11]. A close look at all of the reports of decreasing mortality following the adoption of the SSC guidelines [7] reveals that, in all of them, all aspects of the SSC guidelines have been implemented, and not just the hemodynamic protocol. The reduction in mortality following the implementation of these guidelines therefore may be attributed, in part at least, to the early initiation of effective antimicrobial therapy, which has been shown to play a major role in sepsis outcome [12]. The growing number of reports attesting to the success of the SSC guidelines therefore cannot serve as evidence that the initial hemodynamic resuscitation 'bundle', in and by itself, leads to better survival [13]. In addition, the Rivers single-center study has never been repeated and is therefore the only evidence for the effectiveness of the hemodynamic protocol that is now being recommended for all in the SSC guidelines are based on its perceived physiological flaws and on the possibility that the patients of the Rivers study do not represent all septic patients.
Do the Rivers patients represent all septic patients?
One of the most outstanding findings of the Rivers study is that the mean central venous oxygen saturation (ScvO2) on admission to the ED was less than 50% in both the standard therapy and the EGDT groups [2]. These ScvO2 values are extremely low since the normal ScvO2 is about 75%. Moreover, in septic patients, the ScvO2 is mainly normal or even supranormal due to a reduced oxygen extraction ratio, which is characteristic of septic shock [14,15]. Recent studies have indeed found much higher ScvO2 values in septic shock patients either in the ED or on admission to the intensive care unit (ICU) [16-18]. In two of these studies [17,18], the mean ScvO2 was 72% to 74%; in one of them [18], only 8 out of 125 patients (6%) had an ScvO2 value below 60% and only 1 patient had an ScvO2 below 50%. The septic patients in these studies were also different than the Rivers patients in that the former had lower initial serum lactate levels [16-18], higher central venous pressure (CVP) values (10 rather than 5 mm Hg) [18,19], and lower mortality rates [17-19]. The authors of these recent studies [17-19] have commented that their septic patients were seemingly less critically ill at presentation compared with those of Rivers and colleagues.
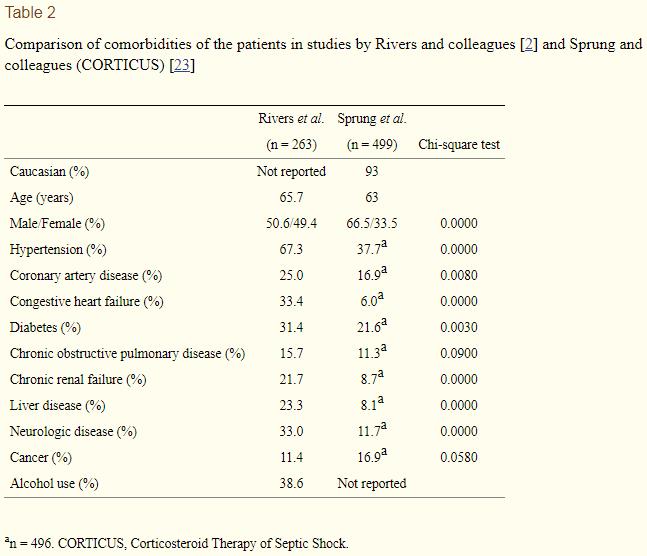
What can account for the differences between Rivers's patients and these other groups of septic patients? One suggested hypothesis is that, in the US system, some patients with sepsis might present much later because of concern about a lack of health insurance and the associated cost of care [19]. The Rivers study was done in the ED of an urban hospital (Henry Ford) in Detroit (MI, USA) and most of the patients who were included in the study may have come from a low socioeconomic background. Very recent literature from the US does emphasize the effects of socioeconomic conditions on sepsis outcome. African-American patients were found to be nearly four times more likely to be uninsured, were more likely to be admitted to the hospital through the ED and the ICU, and had higher mortality for sepsis, most probably due to disparities in disease prevention and care of pre-existing conditions before sepsis onset [20]. Outcome of Americans without insurance who are admitted to the ICU was found to be worse, possibly because 'they are sicker when they seek care' [21]. Males and African-Americans were also found to have a greater frequency of Gram-positive infections, possibly due to specific chronic comorbid medical conditions [22]. The patients of the Rivers study indeed seem to have a very high incidence of significant comorbid conditions. This is evident when these comorbidities are compared with those that were observed in the recent CORTICUS (Corticosteroid Therapy of Septic Shock) study [23], in which all patients had septic shock and evidence of hypoperfusion or organ dysfunction attributable to sepsis (Table 2). In addition, alcohol use, which was reported by nearly 40% of the patients in the Rivers study, was recently found to be independently associated with sepsis, septic shock, and hospital mortality among ICU patients [24].
Recently, it was pointed out that the enrollment of patients with less severe disease, who are less likely to benefit from a drug or treatment, may reduce the usefulness of randomized controlled trial findings for clinical and policy applications [25]. Similarly, it may well be that Rivers's patients had a more severe disease state and a different physiological profile than other populations of septic patients. Thus, the combination of significant comorbidities and a more delayed arrival to the ED of the Rivers patients may have led to a low cardiac output state and, in turn, to the observed very low ScvO2 values. One has to note that the use of the word 'early' in EGDT refers to the time from the patient's admission to the institution of goal-directed therapy and does not necessarily mean that the sepsis itself is of early onset. This differentiation is important since septic shock of early onset was found to be more severe than that of late onset yet was associated with better outcome [26]. The difference between early- and late-onset septic shock may therefore influence clinical trials of therapeutic agents for sepsis and should be taken into account when analyzing the results of such trials [26].
Are the hemodynamic goals of the Rivers protocol siutable to guide resuscitation of all septic patients?
Central venous pressure
The SSC guidelines recommend fluid resuscitation with the aim of achieving CVP values of 8 to 12 mm Hg as the first step in the initial hemodynamic management of severe sepsis or septic shock [10] (Table
(Table1).1). This recommendation is based on the practice parameters for hemodynamic support of sepsis which recommend filling pressures of between 12 and 15 mm Hg for the optimization of cardiac output [3]. These values originate from a study that was done in 1983 in 15 patients undergoing fluid resuscitation for both hypovolemic and septic shock [27]. Since then, however, numerous articles have repeatedly shown that estimates of intravascular volume based on any given level of filling pressure do not reliably predict a patient's response to fluid administration [28,29]. The 2006 International Consensus Conference on hemodynamic monitoring in shock also recommended that preload measurement alone not be used to predict fluid responsiveness [30]. It did add, however, that low values of filling pressures should lead to immediate fluid resuscitation 'with careful monitoring' and that a fluid challenge should be done to predict fluid responsiveness with a goal of obtaining an increase in CVP of at least 2 mm Hg [30]. However, a very recent study done in septic patients has shown that the significance of both CVP and pulmonary artery occlusion pressure (PAOP) to predict fluid responsiveness was poor and that a CVP of less than 8 mm Hg and a PAOP of less than 12 mm Hg predicted volume responsiveness with a positive predictive value of only about 50% [31]. Thus, instituting aggressive fluid resuscitation in patients with low CVP values may lead to fluid overload, which may aggravate pulmonary edema, especially in those patients in whom sepsis is associated with acute respiratory distress syndrome (ARDS) and severe pulmonary dysfunction. This is also true for patients with severe sepsis but without ARDS, of whom more than half have been found to have increased extravascular lung water, possibly representing subclinical lung injury [32]. Hence, we can only join Singer's warning that rapid and large volume loads may lead to iatrogenic fluid overload and that it would be more sensible to give guidelines as to when to use more sophisticated hemodynamic monitoring to better titrate fluid input, rather than 'react post-drowning' [33].
The SSC guidelines go further to recommend that CVP values of 12 to 15 mm Hg be achieved in mechanically ventilated patients or patients with increased intra-abdominal pressure [10] (Table 1). This recommendation is based on a review article [34] that clearly states, however, that filling pressures have a low predictive value in estimating fluid responsiveness during mechanical ventilation and that using them to guide fluid therapy can lead to inappropriate therapeutic decisions. Others recently have claimed that using the CVP to direct fluid resuscitation of patients with elevated intra-abdominal or intrathoracic pressure may place the patient at risk for under-resuscitation with resultant organ dysfunction and increased mortality [35]. (Table1).
Central venous oxygen saturation
Since the CVP was used as a therapeutic goal in both the standard therapy and the EGDT groups in the Rivers study, the use of a target value of 70% for the ScvO2 was, in fact, the main and only difference in the management of these two groups [2]. From the Fick formula, it can be derived that the oxygen extraction ratio is approximately equal to (1 - ScvO2) [36] and that a low ratio will normally be associated with high ScvO2 values. This is why the ScvO2 may not be a reliable parameter to direct therapy in septic patients, since a low oxygen extraction ratio is characteristic of severe sepsis. The combination of low oxygen extraction and high ScvO2 was also demonstrated in other populations of critically ill patients. Rivers and colleagues [37] have described an impairment of systemic oxygen utilization in postarrest cardiogenic shock patients. A similar impairment was found in a group of patients following cardiac surgery in whom abnormally high ScvO2 values were associated with increased serum lactate levels and increased mortality (Perz S, Uhlig S, Reinhart K, Bauer M, unpublished data).
Further evidence for the fact that the ScvO2 values of Rivers's patients are not characteristic of all septic patients can be found in a later study of Rivers and colleagues [38], in which patients of both the standard therapy and the EGDT groups of their original study were combined and then divided into three resuscitation groups. These included (a) severe global tissue hypoxia (lactate of greater than or equal to 4 mmol/L and ScvO2 of less than 70%), (b) moderate global tissue hypoxia (lactate of greater than or equal to 2 mmol/L and ScvO2 of less than 70%), and (c) resolved global tissue hypoxia (lactate of less than or equal to 4 mmol/L and ScvO2 of greater than or equal to 70%) [38]. In a recent multicenter European study [39], we have found, however, that out of 44 septic patients, 10 (23%) had lactate of greater than or equal to 2 mmol/L and ScvO2 of greater than 70%, a 'resuscitation group' category that simply does not exist among Rivers's patients. These findings are more in line with the recent reports of significantly higher ScvO2 values [16-18] than those observed in Rivers's patients.
Thus, for all clinical purposes, a low ScvO2 value is an important warning sign of the inadequacy of systemic oxygen delivery to meet oxygen demands. However, it does not provide information about the reason for this inadequacy, nor does it provide guidance as to the optimal therapeutic approach. On the other hand, a normal or high ScvO2 value does not rule out persistent tissue hypoxia, especially in septic patients. Therefore, very often, the ScvO2 value is unsuitable to guide resuscitation in patients with severe sepsis or septic shock, especially in the ICU (following surgery, trauma, ARDS, and so on), where low oxygen extraction ratios may be more prevalent.
Conclusion
The SSC is one of the most important developments in critical care in recent years. The people who have put this campaign together, as well as Rivers and his colleagues whose work initiated the campaign, should be congratulated for their immense life-saving contribution. Clearly, septic patients should be detected and treated as early as possible since they are at high risk for hemodynamic compromise. Many septic patients, especially those admitted to the ED, may benefit from EGDT according to the SSC guidelines, which currently are being advocated and promoted in the US and internationally in collaboration with public not-for-profit arbiters of the quality of health care [40]. However, we join the concerns that some parts of the 'bundles' of care recommended by the SSC have not been submitted to adequately powered randomized controlled trials and may actually be ineffective or even harmful [33]. Basing international treatment guidelines on the Rivers single-center study would therefore seem premature [41]. This is especially true in view of the fact that the physiological variables that are used by the SSC guidelines to direct EGDT are not suitable for all septic patients and may be misleading in many instances. We have to wait for the results of the new ongoing multicenter studies on the initial hemodynamic management of severe sepsis and septic shock and, until then, exercise caution.
Abbreviations
ARDS = acute respiratory distress syndrome; CVP = central venous pressure; ED = emergency department; EGDT = early goal-directed therapy; ICU = intensive care unit; PAOP = pulmonary artery occlusion pressure; ScvO2 = central venous oxygen saturation; SSC = Surviving Sepsis Campaign.
Competing interests
The author receives consulting fees for serving on the medical advisory board of Pulsion Medical Systems (Munich, Germany) and iMDsoft (Tel Aviv, Israel), and has intellectual-property rights with Drager-Siemens (Lubeck, Germany).
References