Summary
Despite being infrequent, complications of airway management remain an important contributor to morbidity and mortality during anaesthesia and care of the critically ill. Developments in the last three decades have made anaesthesia safer, and this has been mirrored in the equipment and techniques available for airway management. Modern technology including novel oxygenation modalities, widespread availability of capnography, second‐generation supraglottic airway devices and videolaryngoscopy provide the tools to make airway management safer still. However, technology will only take safety so far, and non‐technical aspects of airway management are critically important for communication and decision making during airway crises, acknowledging a ‘cannot intubate, cannot oxygenate’ situation and transitioning to emergency front of neck airway. Randomised controlled trials provide little useful information about safety in this setting, and data from registries and databases are likely to be of more value. This narrative review focuses on recent evidence in this area.
Introduction
Although most airway management undertaken by anaesthetists, intensivists and emergency physicians is routine, uneventful and uncomplicated, when complications do arise they can be catastrophic, with devastating outcomes for patients and clinicians alike 1, 2. These events account for a significant proportion of fatalities 3 and litigation related to anaesthesia 4.
Anaesthesia is increasingly safe, with current estimates of anaesthesia‐related mortality falling almost ten‐fold in the 20 years up to 1990 (3.6 per 10,000 pre‐1970, 0.5 per 10,000 1970–1980s, 0.3 per 10,000 after 1990) 5. The proportion of these deaths that are due to airway complications is unknown, and is likely to vary according to setting. In a 2005 study of > 1 million ASA physical status 1–2 patients, the risk of death or other serious complications from anaesthesia was about 10 per million anaesthetics, with 40% of events related to airway problems 6, whereas another Japanese study reported cardiac arrest in 100 per million anaesthetics, with airway complications and aspiration accounting for 11% of events 7. The 4th National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society (NAP4) reported an incidence of airway‐related death and brain damage of 7 per million general anaesthetics in the UK 1, 2. However, in less well‐resourced settings, it is likely that anaesthesia‐related mortality, including those from airway events, is considerably higher. In Togo, the mortality rate 24 h after surgery was 2.6%, with 93% of deaths judged avoidable; 50% were attributed to anaesthesia, including 30% attributed to ‘respiratory management’ 8.
The nature and incidence of complications related to airway management is well described and has been subject to numerous reviews. It is not possible in a single review to examine the full breadth of developments in this topic. Previous reviews describe complications and failure of airway management and the topics of pulmonary aspiration, airway trauma and respiratory complications of lung ventilation 9, 10. Rather than duplicate data available elsewhere, this narrative review instead focuses on recent literature and, in particular, how this provides information on the prevention of such complications.
Research into complications – why randomised controlled trials are of limited value
A recent editorial commented on the “hegemony of the randomised controlled trial (RCT) as the poster child of evidence‐based medicine”, and the importance of embracing other research modalities 11. This is particularly the case when considering rare complications. The typical airway RCT will be designed with a primary outcome that is a measure of efficacy (e.g. first insertion success with supraglottic airway devices (SADs) or videolaryngoscopy) or, worse, a surrogate of efficacy (e.g. airway leak pressure with SADs, laryngeal view with videolaryngoscopes). Use of this primary outcome measure will lead to a trial of usually 40–100 patients that is powered to detect a clinically relevant difference in performance. These studies are invariably performed in elective, healthy patients, without risk factors for difficult airway management or hypoxaemia, and often performed by expert clinicians. Indeed, it has recently been argued that these are prerequisites for safe and appropriate airway research 12. As a result, there are a profusion of airway RCTs that only provide information about aspects of device efficacy. These studies generally show that most new devices work reasonably well (> 90% efficacy) in these low‐risk settings. Meta‐analyses of these same studies inform us that these devices perform similarly (in these low‐risk settings) 13-15, even when that does not ring true among clinicians using the devices on a daily basis in normal clinical practice.
However, in modern airway management, SADs are used for a variety of situations and patient groups including: the obese; patients with high Mallampati scores and other risk factors for difficult airway management; patients with varying degrees of gastro‐oesophageal reflux; prolonged surgical procedures; abdominal surgery; surgery in lithotomy or prone positions; airway rescue; during cardiopulmonary resuscitation; and as a conduit for tracheal intubation 16. Modern tracheal intubation equipment is used in increasingly comorbid and obese patients. In each of these settings the key decision in choosing a device is safety. It is highly likely that devices determined to be ‘similarly efficacious’ in small RCTs differ in performance when stressed by clinical setting or use in higher risk populations/procedures 17. Randomised controlled trials performed in low‐risk patients are unable to answer questions about safety in complex patient populations or high‐risk settings, and these data must be sought elsewhere.
The role of registries and databases
Databases collect large amounts of information about routine cases, whereas registries more commonly collect data about specialist procedures or subspecialty practice. Databases not only have the potential to represent outcomes of everyday practice but also have limitations. Common limitations include the population captured (single hospital or atypical hospital population), and data collected (if data are collected for billing or administrative purposes it may lead to omissions or perverse associations). Several institutional databases are relevant for exploring complications of airway management:
Relevant registries include:
Fatal case reviews
Case reviews may include local critical event analyses, coronial cases, medico‐legal cases, published case reports (usually describing rarities) or analyses of registries and national audits. Apart from registries and national audits, these involve single cases analysed by individual clinicians and provide limited benefit in terms of learning from thematic analysis and case similarities. Through involvement in all such types of fatal case reviews, common themes can be identified (Fig. 1). It is likely that as much can be learnt from the review of cases of difficulty where difficulty was resolved with a favourable outcome, but that resource is currently rarely examined.

Figure 1
Recognisable events and pitfalls of fatal airway complications.
Epidemiology of major and minor airway events
NAP4 is considered by many to be the current benchmark in terms of quantitative data about the epidemiology of important airway complications 1, 2. It comprised a one‐year national registry of major airway complications arising during anaesthesia and in the ICU or ED that lead to death, brain damage, eFONA, ICU admission or prolongation of ICU stay. This was supported by a numerator survey and in‐depth analysis of each submitted case.
NAP4 reported 46 events per million general anaesthetics (95%CI 38–54) or 1:22,000 (95%CI 1:26,000–18,000). Among approximately 3 million general anaesthetics, there were 16 airway‐related deaths and three cases of persistent brain damage, equating to a mortality rate of 5.6 per million general anaesthetics (95%CI 2.8–8.3) or 1:180,000 (95%CI 1:352,000–120,000) (Table 1).
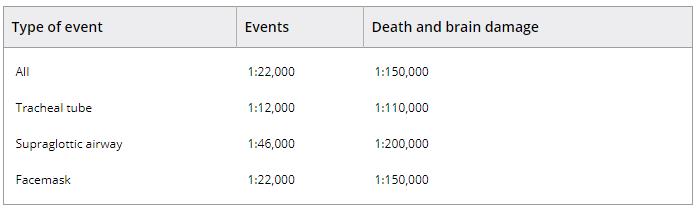
Table 1. Point estimates for airway complications in the 4th National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society 1, 2
Several themes were identified in NAP4:
The project made many recommendations designed to improve individual, institutional and national resilience in airway management. Two years after the publication of NAP4, a survey identified that practice changes had been instituted in response to NAP4 in 98% of UK hospitals 41. The survey used a safety gap analysis (assuming compliance with the recommendations to be a metric of safety) to measure change. The highest safety gaps were in the ICU and ED. The extent to which these safety gaps were closed was 39% closure in anaesthesia, 48% in ED and 59% in ICU.
Huitink et al. adopted a different approach, and attempted to capture all potential and actual significant airway events in a Dutch University hospital's operating theatre over a 60‐day period 42. Cases were identified by daily interviews with clinicians, being volunteered by clinicians and by triggered interviews when the anaesthesia information system detected an episode of oxygen desaturation (SpO2 < 93%). Episodes were divided into airway problems (unavoidable events not leading to harm) and complications (avoidable events with the potential or causing actual harm). Degree of harm was scaled, with ‘serious’ harm equivalent to NAP4 entry criteria. During 2803 general anaesthetics (81% elective, 19% emergency; 75% adult, 25% paediatric) there were 168 (6%) airway‐related complications. Events included: 24 (0.9%) serious complications (events which would have triggered inclusion in NAP4); one death in which eFONA contributed; one CICO; two eFONAs; and 12 unplanned ICU admissions (Table 2). Forty‐four (1.5%) patients desaturated to SpO2 < 93% and eight (0.3%) to < 50%. As a proportion of events, tracheal intubation difficulty accounted for 23%, failed mask ventilation 3%, aspiration 1.8% and laryngospasm 7%. Events were most common in healthy, male patients, in adults aged > 40 years or children aged < 10 years, and in those with an elevated body mass index (BMI) (48% of events in patients with BMI > 26 kg.m−2). Timing of events was at induction of anaesthesia in 69%, during maintenance in 12%, and after surgery in 14%. This study provides an important counterpoint to NAP4, emphasising how NAP4 only examined the ‘tip of the iceberg’; for every case captured by NAP4 there may be another 720 potential airway events occurring that do not progress, for whatever reason. This emphasises the importance of airway vigilance and skills in anaesthetic practice.
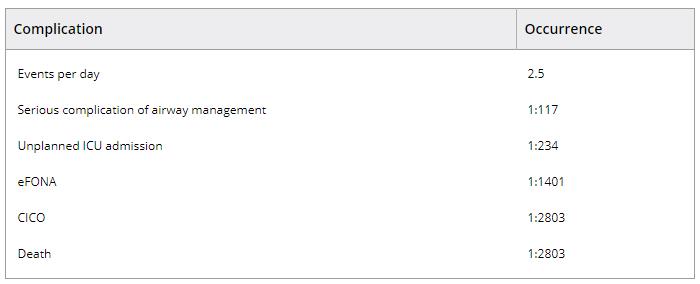
Table 2. Rates of airway complications in Huitink's Dutch study of potential and actual airway complications in a university hospital 42
ICU, intensive care unit; eFONA, emergency front of neck airway; CICO, cannot intubate, cannot oxygenate.
De Jong et al. studied the epidemiology of airway complications in obese ICU patients 43. This study compared two sequential cohorts of obese patients whose tracheas were intubated during anaesthesia (n = 11,035, 19% obese) or in the ICU (n = 1400, 20% obese). Difficulty during tracheal intubation occurred twice as often in obese patients in ICU compared with anaesthesia (16% vs. 8%). More importantly, advanced ‘difficult airway management’ techniques were rarely used in the ICU compared with during anaesthesia (10% vs. 36%), and life‐threatening complications were significantly more common in the ICU than during anaesthesia (41.1% vs. 1.9%, relative risk (RR) 95%CI 21.6 (15.4–30.3); p = 0.01). Among the obese ICU patients with difficult tracheal intubation, the rates of severe hypoxaemia (39%), severe cardiovascular collapse (22%), cardiac arrest (11%) and death (4%) were of great concern. This important study reinforces many of the findings of NAP4 regarding the dangers of airway management in the ICU, issues around preparedness and management of high‐risk patients and the importance of obesity. The study further identified risk factors for difficult tracheal intubation in the obese: Mallampati score 3–4; obstructive sleep apnoea; and reduced cervical spine mobility.
Of note, NAP4 and the studies by Huitink et al. and de Jong et al. all emphasise the association of elevated BMI and adverse airway events. Although obesity may impact on the ease or success of facemask ventilation, SAD insertion, laryngoscopy (modestly 44) and eFONA, its most profound impact is on the speed and extent of hypoxia during routine and difficult airway management, which dramatically reduces the time during which success must be achieved 45. Patients with obstructive sleep apnoea are at even higher risk of increased airway difficulty and airway obstruction leading to rapid hypoxia.
Martin reported a series of 3423 emergency out of theatre tracheal intubations, all performed by experienced operators 27. Tracheal intubation difficulty (Cormack–Lehane grade 3–4 or > 2 attempts) was reported in 10%, and complications (4.2% overall) included aspiration (2.8%) and oesophageal intubation (1.3%). As in Mort's previous work 46, multiple attempts (> 2) were associated with an increase in complications (OR 95%CI 6.7 (3.2–14.2)) as was tracheal intubation in the ED (OR 95%CI 4.7 (1.1–20.4)). The eFONA rate was 1:380. Videolaryngoscopy was not in routine use, and approximately 30% did not receive a neuromuscular blocking agent (NMBA).
In contrast, a Scottish national survey of tracheal intubation performed by critical care doctors of similar experience noted much lower rates of airway complications 47. In this series of 794 patients (92% of whom received NMBA), first‐attempt tracheal intubation success was 91%, despite 6% being Cormack–Lehane grade 3–4; only 1% required more than two attempts. Oesophageal intubation occurred in 2% and there was one eFONA (1:794). Severe hypoxia and hypotension both occurred in approximately 20%.
Failure of routine airway techniques
Defining failure is part of the problem. Most anaesthetists experience airway management failure every day or so; a poor seal is achieved with facemask ventilation; a SAD needs to be repositioned; or tracheal intubation requires more than one attempt or needs patent repositioning to be successful. In most studies of airway device performance, these would be judged ‘failures’. In clinical practice, the anaesthetist moves to another technique and is usually successful, and these minor failures are of little or no consequence in the majority of cases. However, failures such as these are likely to be the first step in many more important and potentially life‐threatening complications.
Failure rates (i.e. inability to perform a technique after multiple attempts) for routine anaesthetic airway management techniques are as follows: facemask ventilation < 1:700; insertion and ventilation via SAD < 1:50; tracheal intubation < 1:1500; CICO < 1:5000; and requirement for eFONA ~ 1:50,000.
Multiple attempts without changing some aspect of technique are illogical, and rarely effective. For example, after failed tracheal intubation, subsequent attempts have a success rate of approximately 20% 48. Multiple attempts also increase the risk of airway trauma and development of airway obstruction, and compromise the chances of success of other techniques 32. Limiting the number of attempts at a given technique is a fundamental aim during difficult airway events.
Several airway techniques have common risk factors for difficulty, including: obesity; reduced mouth opening; Mallampati class 3–4; neck rigidity; and previous neck irradiation. When one airway technique fails, the risk of failure of other techniques is higher than anticipated (‘composite airway failure’ 10). Specific examples include: when tracheal intubation fails, mask ventilation may fail in up to 10% of cases 49; when mask ventilation is difficult, the risk of failed tracheal intubation increases more than 10‐fold 50; and when SAD insertion fails, the risk of difficulty in mask ventilation is increased threefold 29.
In order to minimise the risk of complications, when one technique is predicted to be difficult it is important to focus carefully on assessing the likely ease of other techniques that may be used for rescue.
Avoiding complications
The commonest modes of airway management in anaesthesia are SAD insertion and tracheal intubation 50, accounting for 95% of airway management techniques. These are also, with facemask ventilation, the methods for maintaining the airway in emergencies and crises. Therefore, the main priorities in avoiding airway complications are:
These are also the main principles embedded in the Difficult Airway Society (DAS) 2015 guidelines on difficult airway management 51.
Avoiding hypoxia
The most feared complication of airway management is hypoxic death or brain injury. This is rare during anaesthesia (1:180,000), but more common as a complication of airway management and maintenance in the ICU (approximately 50‐ to 60‐fold higher) or in the ED (approximately 30‐fold higher) 1, 2. NAP4 showed that approximately 40% of such events are initiated by delayed or difficult tracheal intubation. Although many airway algorithms now adopt an ‘ABCD’ approach, oxygenation is not explicitly addressed within this, and it might be useful if this were changed to ‘OABCD’, in order to emphasise the priority of maintaining high circulating levels of oxygen in the blood, both before and during all attempts at airway management.
Previously established pre‐oxygenation practice has included breathing 100% oxygen for 3–5 min via a tight‐fitting facemask. In the critically ill and in the obese (both at higher risk of early and severe hypoxia) 45, both sitting up/reverse Trendelenburg positioning 52 and the use of continuous positive airways pressure have been shown to be beneficial 53.
Recent interest has focussed on the potential for high‐flow nasal oxygen (HFNO) delivered by cannulae at up to 60 l.min−1 to prolong the safe apnoeic time. This technology has been shown to be highly effective during elective apnoeic oxygenation (although less effective in obese patients) 54. However, its value is less certain during RSI, where its use did not improve oxygenation at the time of tracheal intubation 55. In the critically ill, trial designs that include small groups, and control arms with non‐standard methods of pre‐oxygenation 56, have led to literature without clear evidence of benefit. A recent pilot study of the addition of HFNO to non‐invasive ventilation (10/5 cmH2O) in 48 critically ill hypoxic patients, reported a reduction in the incidence of severe hypoxia from 21% to 0%, but no other outcome benefits 57.
There are alternative methods to deliver oxygen during airway management (‘per‐oxygenation’) and these include: use of simple nasal cannulae at a flow of ≥ 15 l.min−1 once the patient is unconscious 58; or buccal oxygen 59. Both prolong safe apnoea time. In paediatric practice, pharyngeal oxygen delivered via the laryngoscope 60 is a well‐established technique, and this might also have utility in the adult population 61.
The present level of evidence indicates that per‐oxygenation should be used for all patients in whom difficult airway management is anticipated, and arguably in all patients undergoing general anaesthesia. Following loss of consciousness, it is logical to continue efforts at per‐oxygenation. This is particularly so for those with high oxygen demand (the obese, critically ill, septic and pregnant) and in those in whom airway management may be predicted to be prolonged. The optimal method of delivering per‐oxygenation has not yet been established and further research is needed to define the safest, most effective and most economical solution.
As no method of pre‐ or per‐oxygenation is effective if the airway becomes obstructed 62, all efforts should be made to ensure a patent airway throughout any period of pre‐ and per‐oxygenation. Patients at particularly high risk of obstruction are the obese, those with pre‐existing airway obstruction and those with obstructive sleep apnoea (most of whom are likely to be undiagnosed 63).
Avoiding complications of SADs
Harm from use of SADs derives mostly from airway failure (failed ventilation) or pulmonary aspiration. Mechanical injury (direct trauma, nerve injury) is considerably less common than when using a tracheal tube. Basic principles should be followed and SADs should only be used according to, and in settings consistent with, manufacturers’ instructions for use. Basic good practice includes avoiding SAD use where there is a clinically significant risk of aspiration (which is an undefined status, recognised by most anaesthetists but open to considerable interpretation). Training in device use should be meticulous, and when used, devices should be tied in place; a poorly seated or poorly performing device should not be accepted, as these are prone to cause problems 1, 10.
However, in deciding the safe limits of SAD practice, many manufacturer's instructions are not specific and most rely hugely on clinical judgement. The breadth of indications for which SADs are now used is enormous, ranging from neonatal resuscitation, to abdominal, cardiac, neurological or prone surgery and even within ICU. Different clinicians will have a wide range of personal views on what is a safe limit 64 and individual clinician skill and experience may contribute to the limits of safe practice. The absolute ‘limits of safety’ are still undefined. It is, however, pertinent to remember that those limits will only be established by passing beyond safe use and contributing to patient harm.
As described above, the current evidence base regarding RCTs of SADs involves small studies performed in low‐risk patients. There have been three meta‐analyses of SADs: LMA®1 ProSeal™ (PLMA; Teleflex Medical, Wayne, PA, USA) vs. LMA Supreme™ (SLMA; Teleflex Medical) (seven studies, n = 666) 13; PLMA vs. i‐gel (14 studies, n = 1104) 14; and SLMA vs. i‐gel® (Intersurgical, Wokingham, UK) (10 studies, n = 860) 15. There has also been a review comparing the PLMA and LMA Classic™ (Teleflex Medical) (nine studies, n = 1436) 65. This equates to a total of 4066 patients in 40 studies, an average of 101 patients per study. With less than 50 patients on average exposed to the novel SAD in each study, it is unlikely that any study provides much evidence regarding safety; if no adverse events occur in a series of 50 cases the lower limit of the 95%CI for such an event is 3/50 (6%) 66. If RCT were set up to examine the risk of aspiration when using the LMA Classic and another SAD in a low‐risk population (baseline risk of aspiration 1:10,000 67), using standard power calculations (power of 90% and a type 2 error of 5%) the study would require 1.3 million patients in each group. Even then there would be a 10% risk of a false negative and a 5% chance of a false positive result.
Evidence of safety of SADs may more usefully be derived from large cases series, ideally in representative populations. For many devices in current use there are no series of > 100 patients.
Theiler et al. reported on 2049 uses of the i‐gel 68. First‐attempt insertion success was 93% and overall success 96%. Median airway seal was 26 cmH2O. Premature removal occurred in 0.75% and failure/complications in 5.4%. Failure was associated with male sex, poor teeth, small jaw and older age. Complications included laryngospasm 1.2%, nerve injury 0.1%, glottic haematoma 0.05%, vagal response and cardiac arrest 0.05%.
Cook reported a series of 1000 PLMA uses by one operator 69. One‐quarter of patients weighed > 90 kg and 17% underwent abdominal surgery (12% laparoscopic, 5% open). The PLMA was inserted and used for ventilation on first attempt in 85% and overall in 99.4%. Of the six failures, two occurred during difficult airway management. Median airway seal was 32 cmH2O. Premature removal occurred in 0.4% and failure/complications in 3.4% patients. Complications included minor airway obstruction not requiring removal 2.7%, failure (need for removal after an initially successful insertion) 0.4% and nerve injury 0.1% 70.
There are two large series of LMA Classic uses, although both provide less detail than the above studies. Verghese and Brimacombe studied 11,910 cases of LMA Classic use reporting a 0.19% failure rate and an incidence of 0.15% for ‘critical airway incidents’ 71. Bernardini and Natalini retrospectively compared aspiration risk with the LMA Classic and tracheal tube and reported three episodes in 38,200 LMA Classic uses and an adjusted OR (95% CI) for aspiration with the LMA Classic compared with the tracheal tube of 1.06 (0.20–5.62) 72. Ramachandran reported on the use of the LMA Unique (ULMA; Teleflex Medical)) in 15,795 cases 29. Failure rate was 1.1%, predominantly due to leak or ventilation failure, and such failures were associated with hypoxia, hypercapnia and airway obstruction. When the ULMA failed there was a threefold increased risk of difficulty in mask ventilation. Those in whom the device failed were at an increased risk of unplanned hospital admission (14%) of whom 5.6% required ICU admission. Risk factors for failed use were male sex, poor dentition, increased BMI and surgical table rotation. Further specialty‐specific series exist for the PLMA 73 and SLMA 74, both in the obstetric setting.
With the current literature and the pre‐existing limitations of RCTs in this setting, especially when compared with the greatly expanded and extended routine practice, clinicians must make choices about SADs based on device design, device material and evidence from benchtop, cadaver 75, 76 and clinical trials not restricted to RCTs 16. There is an increasing consensus (particularly in mainland Europe, north America and Australasia) that routine use of second‐generation SADs (i.e. those designed with the intent of reducing the risk of aspiration 77) is good practice 16. Using this strategy will provide the following performance characteristics compared with the LMA Classic:
However, for the reasons described above, it is not possible to prove that this strategy improves safety or clinical outcomes.
Avoiding failed tracheal intubation and its sequalae
Failed, difficult, prolonged or misplaced (oesophageal) tracheal intubation is the primary event in approximately 40% of major airway events 1, 2, and occurs during the progression of the event in the majority of such incidents. It is also associated with airway trauma and aspiration 1, 2, 32 and precedes, by definition, all cases of CICO. Difficult or failed tracheal intubation may arise because of difficult laryngoscopy (difficulty seeing the laryngeal inlet), difficulty passing the tracheal tube, or both.
An important strategy for avoiding difficult tracheal intubation is avoiding intubation altogether when it is not indicated. The increased performance of second‐generation SADs is one driver to reduced use of tracheal intubation. However, such decisions require significant clinical judgement, and it is likely that the most difficult airway cases will remain predominantly managed by tracheal intubation, whether asleep or awake. Where difficulty is anticipated, tracheal intubation should generally be performed awake and the options now include both fibreoptic and videolaryngoscopic techniques.
There is little robust evidence around the incidence of difficult tracheal tube passage but it certainly occurs. Strategies to reduce this include:
When the above techniques are adopted, difficult tracheal tube passage should become a very rare problem indeed. The problem much more commonly associated with difficult tracheal intubation is, therefore, that of difficult laryngoscopy. Approximately 6% of patients without features predictive of major difficulty will be Cormack–Lehane grade 3–4 at laryngoscopy 82, and in a recent analysis of > 180,000 intubations from the Danish Airway Database, 93% of difficult tracheal intubations were not anticipated 18.
Difficult laryngoscopy is associated with failure of tracheal intubation, multiple attempts, airway trauma and oedema in addition to major airway complications such as hypoxia, aspiration, cardiac arrest, CICO and death 1, 2, 10. Although Cormack–Lehane grade‐4 laryngeal views are most strongly associated with failed tracheal intubation, they are rare and the majority of failures (> 70%) arise from grade‐3a or even grade‐2b laryngeal views 10 (i.e. ‘restricted views’ in Cook's classification 83). Videolaryngoscopy has an important role in both preventing and managing difficult laryngoscopy.
In a prospective study, unanticipated difficulty in tracheal intubation occurred at a rate of 0.1% 42; resolution of these difficulties was achieved with a videolaryngoscope in 52%, with fibreoptic intubation in 14% and with a combination technique in 10%. A Cochrane systematic review (64 RCTs, > 7000 patients, overall moderate quality evidence) reported numerous benefits of videolaryngoscopy vs. direct laryngoscopy for tracheal intubation 84. These included:
The outcomes ‘time taken for tracheal intubation’, ‘hypoxia’ and ‘mortality’ were not analysable due to variations in definitions of these outcomes or lack of data. There was a lack of evidence of impact of videolaryngoscopy on the development of a sore throat, on the number of attempts taken, or in failed tracheal intubation when difficulty was not predicted (OR (95%CI) 0.61 (0.22–1.67)). In pre‐planned sub‐group analyses, there were inadequate data to report on the impact of videolaryngoscopy in specific clinical locations (e.g. ICU or ED) or in obese patients. However, the data showed that benefits of videolaryngoscopy were only statistically significant when used by an operator who was experienced (defined as > 20 prior uses) (OR (95%CI) 0.32 (0.13–0.75)).
The review also provides some evidence of differential performance between different videolaryngoscopes. The C‐MAC® (Karl Storz GmbH, Tuttlingen, Germany) standard blade reduced failed tracheal intubation (OR (95%CI) (0.15–0.68)), but reductions in failure with other devices did not reach statistical significance. Conversely, a recent RCT suggested a modest increase in first‐time success during use of the Glidescope (Verathon) compared with the C‐MAC D‐blade (Karl Storz GmbH), although this difference was not sustained after multiple attempts or in the hands of senior staff 85.
Further studies are required in order to determine the relative value of different videolaryngoscopes and in different clinical settings and locations. In particular, the literature regarding videolaryngoscope use in obstetrics and ICU is particularly unclear. Several recent registry studies have reported increased utility of videolaryngoscopy in the ED setting. Sakles et al. compared the relative success of direct and videolaryngoscopy on first‐attempt tracheal intubation success in 2423 patients (Fig. 2) 86. Videolaryngoscopy outperformed direct laryngoscopy in all settings with an overall OR of success of 2–3 across all patients. The same group also reported that in 460 failed tracheal intubations in the ED, videolaryngoscopy was successful in rescuing tracheal intubation in 82% of attempts, compared with 61.7% of direct laryngoscopy attempts (OR (95%CI) 3.5 (1.9–6.7)) 87. Direct laryngoscopy had a first‐attempt success rate of 58%, with 78% of these tracheal intubation failures rescued on first attempt with videolaryngoscopy 88.

Figure 2
First‐pass success with direct or videolaryngoscopy in 2423 patients for direct laryngoscopy (blue bars) and videolaryngoscopy (orange bars) 88.
The National Emergency Airway Registry (NEAR) has also measured the impact of videolaryngoscopy on emergency tracheal intubations 34-36. In a series of 8000 cases between 1997 and 2002, a change in tracheal intubation technique was required in 5%, failure occurred in 1%, complications occurred in 9% and eFONA in 1:120, increasing to 1:59 for trauma cases 35. Using the C‐MAC videolaryngoscope, the group reported a higher rate of good views (Cormack–Lehane grade 1–2) (93% vs. 80%; p < 0.0001). In those with a poor direct view, videolaryngoscopy improved the view in 78%, and in those with a good view on direct laryngoscopy, videolaryngoscopy worsened the view in 4% 34. More recently, NEAR reported on 17,583 ED tracheal intubations (85% RSI) in USA, Canada and Australia between 2002 and 2012 36. During the study, use of videolaryngoscopy increased from < 1% to 27%; first‐attempt success increased from 80% to 86% as videolaryngoscope use increased.
As a note of caution, there are two recent studies suggesting harm associated with videolaryngoscopy. Both involved critically ill patients, and there is a paucity of good quality evidence in this area. In a well‐designed study, Yeatts et al. examined the impact of GlideScope use on outcome in a major trauma centre 89. Although GlideScope use had no effect on overall survival (p = 0.43), in a retrospectively identified cohort of patients with severe brain injury, its use was associated with increased mortality (p = 0.047). Use of the GlideScope was associated with an increased time for tracheal intubation and increased rates of hypoxia. The MACMAN study, compared use of the McGrath™ MAC (Medtronic, Minneapolis, MA, USA) videolaryngoscope with direct laryngoscopy in critical care 90 Use of the videolaryngoscope was associated with improved glottic view, but not with increased speed or success at tracheal intubation and after post hoc analysis, videolaryngoscopy was associated with an increase in severe life‐threatening complications (9.5% vs. 2.8%, p = 0.01). Although both studies may cause concern among proponents of videolaryngoscopy, the study findings have several potential explanations. In the study by Yeatts et al. the finding was both a post hoc analysis and statistically fragile. Notwithstanding that, if use of videolaryngoscopy slows down tracheal intubation this may have an important impact in the critically ill. This is perhaps an argument for favouring (for routine use) a videolaryngoscope that can be used for both direct and videolaryngoscopy rather than an angulated videolaryngoscope which cannot be used directly; an angulated videolaryngoscope could be reserved for genuinely difficult laryngoscopy 91. In the MACMAN study, although the study was generally well designed, the protocol had a few anomalies which included the use of predominantly inexperienced tracheal intubators, and uncertainty over the extent of training with the videolaryngoscope. The McGrath MAC videolaryngoscope has also been studied in routine anaesthesia, without showing any apparent benefit over direct laryngoscopy 92. It would be of value to repeat this study using a device of proven benefit in the anaesthetic setting and used by operators trained and expert in the device.
Cannot intubate, cannot oxygenate
The term CICO is increasingly used for the previously described ‘cannot intubate, cannot ventilate’ (CICV). It better describes the problem and the priority and, therefore, is advocated 93.
Cannot intubate, cannot oxygenate is more common during emergency airway management, in patients with head and neck cancers and in obesity, trauma, obstetrics and locations outside the operating theatre 1, 2. In the elective setting, it occurs most commonly after multiple attempts at tracheal intubation or other airway manoeuvres in patients in whom ventilation was previously possible 1, 2, 32.
Avoiding CICO
Although pre‐operative assessment is designed to detect an increased risk of airway difficulty, in a recent analysis of > 180,000 intubations from the Danish Airway Database 94% of episodes of difficult mask ventilation were not anticipated 18. Kheterpal et al. identified that difficult facemask ventilation occurred in 5% of cases and difficult laryngoscopy in 5.8% (n = 176,679) 30. Difficult facemask ventilation was almost twice as frequent when NMB was not administered and administration of NMB was noted to improve mask ventilation in 3% of cases, but never to worsen it. This is compelling evidence of the value of muscle paralysis when trying to optimise mask ventilation. Of note, if difficulty in mask ventilation and tracheal intubation are independent of each other, one would anticipate an incidence of 0.29% (1:330); however, the authors reported an incidence of difficulty in mask ventilation with difficult laryngoscopy of 0.4% (1:250), suggesting that the two factors interact. Of 678 patients who had both difficult mask ventilation and difficult laryngoscopy, 461 were rescued with direct laryngoscopy alone or with a bougie, 163 with a videolaryngoscope, 35 with a fibreoptic technique and six with an LMA Fastrach. In another large series of 53,000 anaesthetics, there were 77 (0.15%, 1 in 690) cases of impossible mask ventilation 26. Factors predictive of this were: neck radiation; male sex; sleep apnoea; Mallampati score 3–4; and a beard). In 25% of the cases there was associated difficult laryngoscopy (1:2800 overall).
Considerable discussion has taken place in the anaesthetic literature recently on how best to manage CICO, and which eFONA technique to use. Perhaps a more useful strategy would be to increase efforts on those techniques that are likely to prevent CICO.
These are central to the DAS 2015 guidance 51 but can be expanded:
These guidelines advocate the use of videolaryngoscopy and second‐generation SADs when difficulty occurs. This begs the question, as they are considered more effective and safer, as to why they are not used as first choice for all patients. This will likely improve speed, efficiency and safety of airway management. In some hospitals, there is a move towards exactly this practice.
Cognitive aids such as physical representations of algorithms or aide memoires are likely to improve compliance with guidelines 94, 95, but their utility is impacted by design, and linear algorithms appear to be better followed than others 96. In this respect the DAS 2015 guidelines are well constructed. Despite this, it is uncertain whether algorithms help in a crisis and augmentation by, for instance smart‐phone apps 97 or read‐aloud action cards, may further enhance completeness of performance. In a simulation study, the use of read‐aloud cards significantly increased compliance with the best practice steps of eFONA, but slowed the procedure 98. This area remains ripe for research.
The Vortex approach© originates from Australia, and is an intuitively appealing approach to any evolving airway crisis. It divides airway management into a ‘green zone’ of safety and a ‘vortex’ of progressive failure culminating in eFONA for management of CICO 99 (Fig. 3). Circling around the vortex are the three airway options: facemask ventilation; SAD insertion; and laryngoscopy. The Vortex approach allows a maximum of three attempts at each technique while the patient is in the green zone, but rapid transition to eFONA if the vortex is entered or after three attempts at each technique. If one ‘best attempt’ at each of the rescue techniques is attempted while in the green zone, further attempts are not mandated. The cognitive aid is designed to ensure focus on the key parts of airway rescue, while avoiding delay, task fixation or cognitive overload. Despite its appeal, the Vortex approach has not undergone formal evaluation to demonstrate whether theoretical benefits are realised in clinical practice.

Figure 3
The Vortex approach© to airway management (vortexapproach.org; reproduced with the permission of Dr Nick Chrimes).
Managing CICO
Perhaps the most important aspect of managing CICO is the decision to manage it. Death after CICO is notably more common due to failure to act than because of procedural complications 1, 2, 32. Although minor complications of the technique are common, fatal complications are very rare. The Australian and New Zealand College of Anaesthetists has been progressive about this issue, both in publishing a robust document regarding the transition to CICO 100 and in mandating that management of CICO is practiced by anaesthetists on a biennial basis 101.
It is now standard practice to recommend both full neuromuscular blockade and an attempt at insertion of a SAD 1, 2, 51. The CICO situation should be explicitly declared and there should be immediate transition to eFONA 51. In an efficient setting, this will have been anticipated (e.g. through use of the Vortex approach) and equipment will be available, open and prepared. If SAD insertion has not been attempted, this should be done immediately, as it will rescue most cases of CICO 102.
Despite much debate and many strong opinions, the optimal technique for eFONA is not known. In the UK, the DAS guidelines are generally supportive of the scalpel‐bougie‐tube technique 51, whereas in Australasia the narrow‐bore needle is generally favoured 103.
NAP4 is often quoted as supporting the arguments put down by those favouring the scalpel‐cricothyroidotomy technique 1, 2. This is based on a misinterpretation of the NAP4 data and recommendations. In NAP4, there were 80 eFONAs: 58 in the operating room; 15 in the ED; and seven in ICU. Anaesthetists were considerably more likely to choose a cannula‐based technique, and in the operating theatre 15 out of 25 attempts performed by anaesthetists failed. Overall failure rate with cannulae (narrow and wide bored) was 65% (22 out of 34). Technical, equipment and operator failings all contributed. A total of 45 ‘surgical or scalpel eFONAs’ were performed, and 44 (98%) were successful in re‐establishing the airway, although not all patients survived. However, it is essential to note that almost all surgical eFONAs were performed by surgeons, and in this setting the anaesthetist often maintained the airway and oxygenation from above. The surgical procedure was often prolonged, in some cases up to an hour in length 104. In contrast, needle techniques used by anaesthetists were generally in a perimortem situation and other efforts at oxygenation had to be abandoned. Overall, NAP4 does not inform us whether the scalpel technique is more effective in the hands of anaesthetists. Given the rarity of such events it is unlikely that further useful information will emerge without registry‐based data collections. Such databases are under development in the UK and Australasia.
More important than NAP4 in this discussion is the review by Duggan et al. published in 2016 105. This systematically examined efficacy and complications of ‘transtracheal jet ventilation’ (more correctly termed ‘high pressure source ventilation’) 106. The analysis indicated reasonably high rates of success of high pressure source ventilation in the elective setting, but extremely high failure and complication rates when the same technique was used in an CICO emergency (42% device failure, 32% barotrauma and 51% overall complication rate). The technique was associated with failure and the development of subcutaneous emphysema that then hampered alternative rescue techniques.
The Danish Airway Database recently reported results of eFONAs from a cohort of approximately 450,000 general anaesthetics over a 7‐year period 24; eFONA occurred in 1 in 1700 anaesthetics. One‐third of cases were not predicted to be difficult tracheal intubations. Approximately half the cases occurred during ear, nose and throat (ENT) surgery, with an almost threefold higher incidence (1:625), and ENT surgeons performed two‐thirds of the procedures. Currently recommended techniques to manage CICO (i.e. SAD insertion and paralysis) were used in a quarter and half of patients, respectively. Anaesthetists performed only a very small number of procedures, but failed in half of those they attempted. Remarkably, despite these shortcomings, the authors reported that no patient died or developed brain damage as a result of the airway event.
Where a narrow‐bore cannula technique is used for eFONA, active expiratory assist provides a new method to provide normal ventilation. Using the Bernoulli principle, the Ventrain (Ventinova, Eindhoven, the Netherlands) device facilitates active expiration via a narrow cannula, thus enabling ventilation at conventional rates and achieving normal minute volumes 107. The technology has been extensively used in benchtop and animal models 107-109. Recently there have been reports of its use in the elective clinical setting 110 and in paediatric airway rescue 111. The technology provides a significant potential benefit when CICO is managed with a narrow‐bore cannula, and more data are awaited.
Finally, the role of sugammadex in management of a CICO situation has been debated. In a minority of such situations, where neuromuscular blockade is contributing to or causing the airway obstruction, sugammadex may be of value. However, sugammadex will not reverse the hypnotic elements of anaesthesia, will not reverse mechanical airway obstruction 112, may cause laryngospasm 113, 114 and if CICO persists it will be necessary to re‐paralyse the patient with a non‐aminosteroid NMB before proceeding to eFONA 51.
Conclusions
Avoidance of major airway complications during routine and emergency care requires avoidance of hypoxaemia, preservation of a clear airway and prevention of pulmonary aspiration of gastric contents.
Anaesthesia has become increasingly safe over the last few decades, arguably as a result of improved monitoring and equipment. In airway management, there have been enormous technological advances and the available equipment has the potential to make every anaesthetic safe from an airway perspective. With the advent of new modalities of oxygen delivery that enable effective per‐oxygenation, widespread availability of capnography 115, second‐generation SADs and videolaryngoscopy, airway management has the potential to become safer still. Despite this, fatalities from failed airway management continue to occur at regular intervals in most countries.
Assessing safety and the impact of interventions is unlikely to be revealed by RCTs that are primarily focussed on efficacy. It is therefore likely that databases and registries will provide data on safety in the future.
Technology can only provide safety benefits when it is used, and used optimally. It is likely that future improvements in safety will be brought about by implementing the technology we already have and using a human factors approach to ensure that it is used to its best effect. This applies particularly to areas such as communication and decision making during airway crises, transitioning between techniques and especially during management of CICO.
Acknowledgements
TM's department of Anaesthesia has received free or at‐cost airway equipment from numerous companies for evaluation or research. TM does not have any financial interest in any airway company. He has spoken at a Storz GmbH meeting about airway management and attended a Covidien expert day, and was not paid on either occasion. No other competing interests declared.
Note
1 LMA is a registered trade mark of The Laryngeal Mask Company Ltd, an affiliate of Teleflex Incorporated.
References
1Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1 Anaesthesia. British Journal of Anaesthesia 2011; 106: 617–31.