Abstract
Septic shock in children is associated with high mortality and morbidity. Its management is time-sensitive and must be aggressive and target oriented. The use of clinical assessment alone to differentiate between cold and warm shock and to select the appropriate inotropic and vasoactive medications is fraught with errors. Semi-quantitative and quantitative assessment of the preload, contractility and afterload using non-invasive tools has been suggested, in conjunction with clinical and laboratory assessment, to direct shock management and select between vasopressors, vasodilators and inotropes or a combination of these drugs. This review aims to describe non-invasive tools to assess the hemodynamic status in septic shock including echocardiography, trans-thoracic/trans-esophageal Doppler and electrical cardiometry. As septic shock is a dynamic condition that changes markedly overtime, frequent or continuous measurement of the cardiac output (CO), systemic vascular resistance (SVR) and other hemodynamic parameters using the above-mentioned tools is essential to personalize the treatment and adapt it over time. The different combinations of blood pressure, CO and SVR serve as a pathophysiological framework to manage fluid therapy and titrate inotropic and vasoactive drugs. Near infrared spectroscopy is introduced as a non-invasive method to measure end organ perfusion and assess the response to treatment.
Keywords: Hemodynamics, Monitoring, Septic shock, Pediatric, Trans-esophageal Doppler, Echocardiography, Cardiometry, Near infrared spectroscopy, Trans-thoracic Doppler
Core tip: We have reviewed noninvasive tools, such as echocardiography, Doppler and electrical cardiometry used to evaluate the hemodynamic status and response to treatment of children with septic shock. As septic shock is a dynamic condition that changes markedly overtime, we suggest a practical approach to guide patients’ management by assessing cardiac output, preload, and afterload using these methods. Near infrared spectroscopy is described as a method to assess end organ perfusion.
INTRODUCTION
Sepsis is a systemic illness following invasion of the body by an infectious agent, to which the body reacts by releasing a storm of inflammatory mediators including cytokines, interleukins and many others, constituting the systemic inflammatory response syndrome (SIRS). Unfortunately, these mediators also depress the myocardium and the vascular tone, increase vascular permeability resulting in fluid leakage and hypovolemia. These changes affect body organs particularly the kidneys, brain, liver, lungs and coagulation system, leading to inadequate tissue and organ perfusion with insufficient supply of oxygen and nutrients to them and defective removal of metabolic wastage. If not early corrected, it will lead to intense cellular damage with multi-organ system failure and ultimately death.
Septic shock develops when sepsis affects the cardiovascular system resulting in different types of shock states, depending on the underlying pathophysiology. On one hand, sepsis can cause cardiac depression with (CO) which is the product of heart rate (HR) and stroke volume (SV). As blood pressure is the product of CO × (SVR), this low CO state caused by shock triggers a reactive increase in the SVR to maintain an adequate BP, to keep the vital organs well perfused at the expense of non-vital organs such as the skin. This results the extremities becoming cold, hence the name of “cold shock”. This high SVR will lead to increase in afterload with further decrease in CO leading to a narrow pulse pressure. Cold shock is more commonly seen in infants and children than in adults. While the latter can double their HR to compensate for a falling CO, children have a much narrower margin to increase their HR and rely instead on intense peripheral vasoconstriction to maintain an adequate BP. The alternate mechanism for shock, more often seen in adults, is vasodilation and decreased SVR. Intense vasodilation will make the skin warmer, hence the term “warm shock”. The body tries to increase the falling CO by increasing the cardiac contractility and HR, leading to bounding pulses and a hyperdynamic state.
These shock states either cold or warm could hap–pen with much overlap and variability overtime thus, the classification of shock either pure “cold” or “warm” has been shown to be prone to errors. Normal BP in a patient with septic shock is not necessarily assuring as it does not always equate adequate vital organ perfusion. Although tissue and cellular impairment may cause altered consciousness level, tachypnea, tachy- or brady-cardia, hypo- or hyper-thermia, decreased urine output, with increased blood lactate and metabolic acidosis, physical examination and laboratory testing alone are not sufficient for the proper management of septic shock[1].
Traditional classification of shock state includes: (1) hypovolemic; (2) cardiogenic; (3) distributive; and (4) obstructive shock. In septic shock, all these states could happen at different degrees at the same time due to variable effects on preload, afterload and contractility. Hypovolemia results from a combination of relatively decreased blood volume caused by vasodilation, increased insensible water loss and increased capillary permeability to the interstitial and third spaces fluid pooling. A depression of the myocardial function by bacterial toxins and inflammatory cytokines may also occur. Septic shock can also present as distributive shock due to peripheral vasodilation or as obstructive shock from diffuse small end-vessel thrombosis. Therefore, adjunctive objective hemodynamic parameters are needed to assess the cardiovascular condition.
Once shock is recognized, the goal of therapy is to improve tissue oxygen delivery (DO2), which is the product of CO multiplied by arterial O2 content (CaO2), i.e., DO2 = CO × CaO2, by improving SV and therefore CO, perfusion pressure and O2 supply. The left ventricular SV depends on the preload, contractility, afterload and HR. Successful therapy should target a normal HR and BP (adjusted for age), capillary refill time of ≤ 2 seconds, adequate peripheral pulses, normal temperature of extremities, normal consciousness level, urine output of more than 1 mL/kg per hour, and a central venous oxygen saturation (ScVO2) ≥ 70%[2]. These targets must be achieved within a short “golden” time frame to avoid multi-organ failure and death[3]. The response to therapy should be frequently monitored, in addition to the essential clinical and laboratory assessment, by objective tools for prompt time-directed management. Invasive tools -mainly through pulmonary artery catheter (PAC) - are more accurate than the noninvasive ones; however, they are time consuming, with special technical difficulties and complications in the small sized infants and children. These obstacles favor the use of noninvasive tools, which should be bedside and user-independent with fast response time to the ongoing hemodynamic changes. They are crucial to guide selection and titration of the most appropriate therapy, including fluid boluses and cardiovascular medications, and to monitor its effectiveness.
CHALLENGES IN THE MANAGEMENT OF SEPTIC SHOCK
Left ventricular SV (and therefore CO) is improved by optimizing the preload, contractility and afterload. However, as up to 50% of patients are fluid refractory[4], their management must be directed towards optimizing contractility and afterload. The pediatric surviving sepsis campaign (SSC) 2012 guidelines recommend classifying patients with fluid refractory shock as cold or warm based on clinical assessment, and to proceed accordingly by administering dopamine/epinephrine to cold shock, or norepinephrine to warm shock respectively. However, such subjective classification of shock as warm or cold was found to be inaccurate, with up to 66% of shock diagnosed as being cold shock by experienced clinicians were found to have vasodilation by invasive measurement[5]. It is therefore recommended to institute instead a therapy guided by the hemodynamic monitoring, as detailed in the section titled “Suggested therapy guided by hemodynamic monitoring”. Septic shock also has various hemodynamic profiles, with children presenting with different combination of changes in CO and SVR that rapidly change over time[6,7]. Mortality was observed to be high in patients with septic shock with persisting low CO after fluid resuscitation[7,8]. Mortality has also been shown to be more frequently related to patients with low CO rather than with low SVR[9].
The management of septic shock is further complicated by the fact that the medications used, including inotropes, vasopressors and vasodilators, act differently on various receptors at different doses. Selecting the appropriate vasoactive medications and monitoring their effect for titration requires frequent measurements of the CO and SVR in addition to subjective clinical assessment. Relying exclusively on the clinical assessment by feeling the temperature of the patient extremities, capillary refill time or blood pressure, which is not a good marker for end organ perfusion, is prone to error. Prompt and accurate assessment of preload, contractility, afterload and tissue perfusion, preferably non-invasively, is therefore essential. The available non-invasive tools and the hemodynamic parameters that they measure are summarized in (Table (Table11).
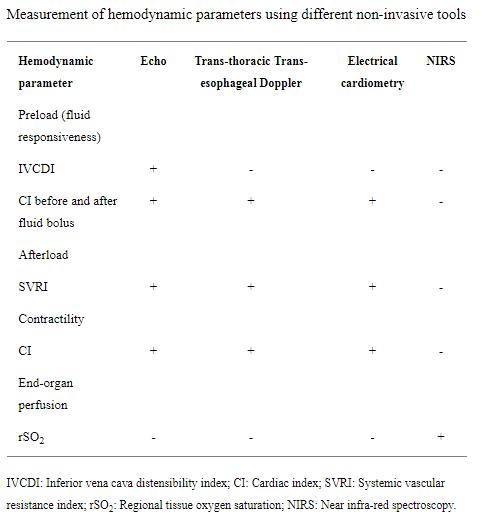
Table 1. Measurement of hemodynamic parameters using different non-invasive tools
Assessment of preload
The pediatric SSC 2012 recommends initial management of septic shock with aggressive fluid therapy, which may exceed 60 mL/kg in the first hour until either improvement of perfusion occurs, or the appearance of rales or hepatomegaly[2]. In 2017, The American College of Critical Care Medicine endorsed the SSC recommendations but advised to assess patients for rales and hepatomegaly before initiating fluid boluses[10].
Fluid bolus therapy is essential for improving SV and hence cardiac output (CO = SV × HR) until a certain limit according to the Frank-Starling curve (Figures 1 and 2). At the steep limb of the curve, the SV increases in response to fluid boluses (fluid responsiveness), but no further benefit is observed when extra fluid is given at the flat limb of the curve. Furthermore, these extra fluids become hazardous, as they will accumulate in various body tissues including the lungs and the liver manifesting clinically as audible rales and palpable hepatomegaly respectively. These changes are detected clinically only after the patient had already received extra fluids and lost a valuable time in the critical initial window for the management of shock. These extra fluids will also extend the patient time on mechanical ventilation, PICU length of stay and increase the risk of morbidity and mortality[4]. Moreover, it was found that as much as 50% of patients presented with septic shock will not be fluid responsive from early on due to initial myocardial depression and changed sensitivity to adrenergic hormones[4,11]. Because of all these factors, there is a definite need for bedside non-invasive tools to assess fluid responsiveness and prevent overload and its hazards.
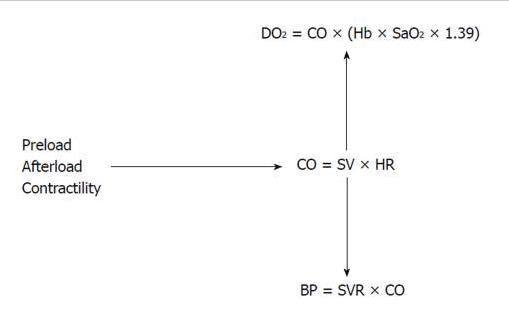
Figure 1. Interplay of hemodynamic parameters. The interplay among the preload, the afterload and the cardiac contractility determines the CO. Cardiac output is essential to maintain a good blood pressure and tissue oxygen delivery. DO2: Systemic oxygen delivery; SaO2: Arterial oxygen saturation; Hb: Hemoglobin level; CO: Cardiac output; SV: Stroke volume; HR: Heart rate; BP: Blood pressure; SVR: Systemic vascular resistance.
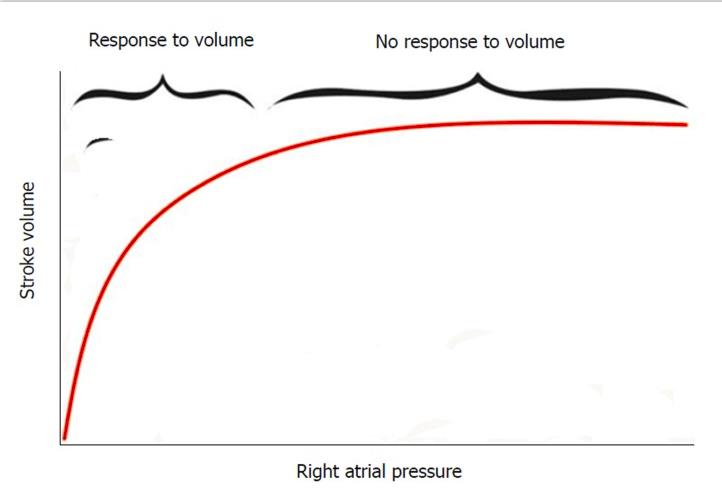
Figure 2. Frank-Starling curve depicting the relation between preload (right atrial pressure) and stroke volume. At the the steep limb of the curve, there is improvement of stroke volume in response to fluid boluses while there is no more response to the fluid boluses at the flat limb of the curve.
Inferior vena cava distensibility index: Distensibility index is a bedside noninvasive parameter for the evaluation of preload volume status and fluid responsiveness, measured by ultrasound. Inferior vena cava (IVC) has no valves and is distensible; its size is easily changeable by changes in blood volume and intrathoracic pressure. IVC Distensibility index is the result of maximum IVC diameter during inspiration minus minimum IVC diameter during expiration divided by the minimal IVC diameter during expiration [IVC distensibility = (maxIVCinsp – minIVCexp)/minIVCexp]. IVC distensibility index has a great value for the assessment of fluid bolus responsiveness in septic shock patients; values more than 18% indicate fluid responsiveness with 90% sensitivity and 90% specificity[12]. However, there are many pre-requisites that must be met to guarantee precise values. Patients must be relaxed and on mechanical ventilation in order to control intrathoracic pressure. They should not be on a trigger mode to avoid spontaneous efforts, which may cause unpredictable changes in the tidal volume and intrathoracic pressure and should be receiving low positive end-expiratory pressure (PEEP) as high PEEP may decrease the delta change in IVC diameter. Patients should not also have any medical condition associated with, or complicated by, increased intra-abdominal pressure[13]. It is also imperative that the attending intensivist possesses the required ultrasonography skills and experience to perform the measurement.
Functional echocardiography, Doppler and cardiometry: They are used for serial measurements of CO and SV to decide the initial resuscitative fluid bolus therapy and assess the response to repeated volume administrations before reaching the flat limb of the Starling Curve. An increase of SV (and so CO) by 10%-15% indicates fluid responsiveness in septic shock[14].
Assessment of CO
Cardiac output is the result of the overall effect of preload, afterload and cardiac contractility. Thermodilution, through a pulmonary artery catheter (PAC), is the gold standard method for measurement of CO, but it is invasive, time consuming and impractical in infants and small sized children due to technical difficulties and risk of complications. Various minimally invasive and non-invasive methods have been developed for a bedside measurement of hemodynamic parameters including Doppler based approaches, electrical cardiometry and bio-reactance.
As in children CO depends on their size, it is imperative to quantify the cardiac index (CI) instead, to remove the influence of different body sizes and to provide a reference number suitable for children of all sizes. Cardiac index L/(min.m2) is the product of CO divided by the body surface area (CI = CO/BSA). In patients without septic shock, a CI of more than 2 L/(min.m2) is considered adequate[15]. However, in those with septic shock, it is recommended to aim a CI of 3.3 - 6 L/(min.m2)[7,8] to compensate for the impaired SVR in order to maintain a good perfusion pressure for best outcome. In 2017, the American College of Critical Care Medicine has endorsed the SSC 2012 recommendation, in the septic shock management algorithm, to measure the CI in fluid-resistant and catecholamine-resistant shock late at the end of that algorithm[10]. This sequence of targeting the CI, after the patient had already received fluid boluses and cardiovascular shock medications, might be intended for settings with no intensive care facilities. In advanced PICU settings, CI monitoring should be considered early on for proper decision on fluid boluses and further choice of cardiovascular shock medications.
Two-dimensional transthoracic and transesophageal echocardiography: Unlike transesophageal two-dimensional (2-D) echocardiography, transthoracic 2-D echocardiography is noninvasive and both methods reliably measure the SV and therefore the CI and systemic vascular resistance index (SVRI). SV is considered as the volume of a virtual cylinder of blood pushed out of the heart with each beat through its left ventricular outflow tract (LVOT). The base of this cylinder is the LVOT area and its height is the VTI (velocity time integral), which is the velocity of blood in systole moving through the LVOT (Figure 3). Accurate measurement of the SV by this method is time consuming, needs a qualified operator and requires manual calculations. Hence, 2D-echocardiography is an impractical method to use for frequent, serial hemodynamic assessment of critically ill children.
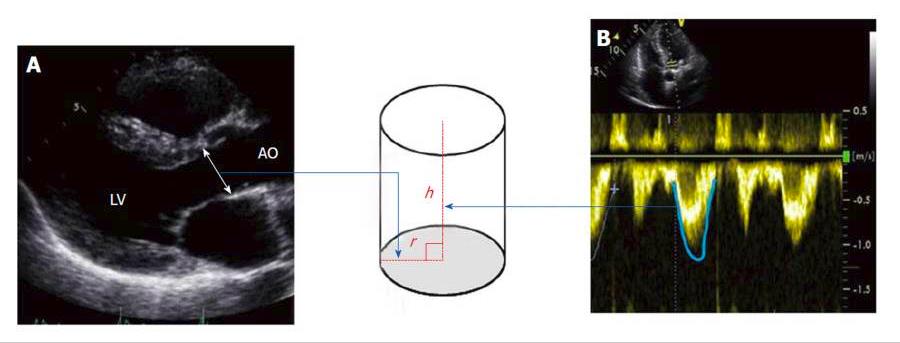
Figure 3. Stroke volume is considered as the volume of a virtual cylinder of blood ejected each beat out of the heart at its left ventricular outflow tract. The base of this cylinder is the LVOT area (area = π.r2) π = 3.14, r is the radius and its height (h) is the velocity time integral (VTI), which is the velocity of blood in systole moving through the LVOT. So, SV will be the net result of π (LVOT diameter/2)2 × LVOT VTI. The diameter of the LVOT is measured using a parasternal long axis view during systole (A) and the VTI is measured with continuous wave Doppler (B). LVOT: Left ventricular outflow tract.
Transesophageal and transthoracic Doppler: The SV is calculated using the same principle as in 2-D echocardiography; the blood velocity is measured in the descending aorta in case of transesophageal Doppler devices and in the ascending aorta or pulmonary artery in case of transthoracic Doppler. The cross-sectional area of the corresponding vessel is calculated by a built-in nomogram based on the patient’s age, gender, weight and height that are fed to the machine[16]. Both machines have an inbuilt algorithm that provides calculations for hemodynamic parameters like CI, SVRI, stroke volume variability and corrected flow time (FTc). The last two parameters are particularly useful in assessing and monitoring preload or ventricular filling. A minimal training is required to adequately place the probe in order to display the best Doppler signal seen on the screen and to hear it through the machine. Doppler based methods are not as accurate as echocardiography, but they are easy to use and they provide trends of the measured values that are valuable in the management of shock. The transesophageal Doppler allows a continuous measurement of the CO in the descending aorta but does not consider the flow in the aortic arch arteries, which represents 30% of the CO. Despite these limitations, its use was found to decrease complication rates and hospital stay in perioperative outcome studies[17]. Although transthoracic Doppler does not offer continuous monitoring of the hemodynamic parameters, the machine is portable, bedside and easy to use allowing frequent monitoring in case of shock. Validation of transthoracic Doppler in children against the gold standard PAC thermodilution method has shown conflicting results. A study in 24 children undergoing cardiac catheterization found that transthoracic Doppler CO measurements were not reliable (Bias -0.13 and precision 1.34 L/min)[18], while another similar study on 31 children demonstrated reliable CO measurements (Bias 0.1 L/min and 95% limits of agreement of -0.6 to +0.9 L/min)[19].
Electrical cardiometry: Electrical cardiometry is a recent non-invasive continuous CO monitoring tool. Its idea is based on its precedent electrical impedance. Four electrodes are attached to the left neck and left lower chest sides. A low magnitude (2 mA), high frequency (30-100 KHz) alternating electrical current (AC) of constant amplitude is applied through the outer electrodes and the resulting voltage is received by the other inner electrodes. The ratio of the detected voltage to the applied current defines bio-impedance.
The theory behind it is that during systole, red cells flow in a parallel manner allowing the electrical current to flow easily thus increasing “electrical velocity” and decreasing impedance. During diastole, red blood cells are arranged randomly, hence impeding the electrical current (increased impedance) and decreasing electrical velocimetry[20]. The changes of impedance overtime are integrated in a complex algorithm that allows to measure CO along with essential hemodynamic parameters including preload (Thoracic Fluid Index), afterload (SVR) and CO, in addition to many other hemodynamic parameters that are beyond the scope of this manuscript. Electrical cardiometry is FDA approved and validated for use in neonates[21], children[22] and adults[23]. A metanalysis in 2016, including 20 studies and 624 patients comparing the CO measurement accuracy by using electrical cardiometry with other noninvasive technologies in pediatrics, demonstrated that electrical cardiometry was the device that offered the most correct measurements. However, there was a high heterogeneity among the individual studies[24]. Furthermore, the accuracy of cardiometry may be affected by severe tachycardia or bradycardia, aortic regurgitation[25], chest wall edema, and high frequency ventilation[26].
Bioreactance: Bioreactance is a modification of thoracic bioimpedance. It needs attachment of two dual electrodes on both sides of the chest and so, tracks the phase of the electrical currents traversing the chest. The underlying scientific phenomenon is that the higher the cardiac SV, the more significant these phase shifts become. Kupersztych-Hagege et al[27] (2013) have shown that bioreactance is not reliable for estimating CO and it could not predict fluid responsiveness in critically ill patients.
Assessment of afterload
Afterload is assessed by measuring the SVR. The resistance (R) in any circuit is the gradient of pressure (∆P) divided by the flow (R = ∆P/Flow). SVR is the gradient between the mean arterial pressure (MAP) at the exit of the left ventricle and the pressure at the entrance of the right atrium (CVP) divided by the flow, which is the CO; [SVR = (MAP – CVP)/CO]. Including body surface area in reporting SVRI[(MAP – CVP)/CI], makes its value independent of body size. SVRI is further multiplied by 80 to convert the results from Woods unit to dyn·s·cm−5 unit. There are now available reference ranges of CI and SVRI according to patient age. Cardiac index and SVRI include the SV in their calculations (Figure (Figure1),1), making SV the corner stone for these measurements. Echocardiography and Doppler based methods, as described earlier, are used to measure SV and calculate both CI and SVRI.
Although there is still no clear evidence that monitoring CI and SVRI improves the outcome of children in septic shock[14], it makes physiological sense and provides intensivists objective parameter to adequately select and guide cardiovascular shock therapy.
Assessment of end organ perfusion
The CO, BP and SVR cannot inform about the end organ perfusion because the CO is not evenly distributed among all organs due to the inhomogeneous vascular resistance across the body. The effect of the commonly used vasoactive medications is not always predictable and may vary at different doses. Concurrent PaCO2, PaO2 and hemoglobin level may affect cerebral oxygen delivery independent of the CO and SVR. Medical conditions affecting oxygen consumption like fever, irritability and thyroid hyper function may also increase oxygen extraction and shift the balance towards oxygen and metabolites deficit.
Near infrared spectroscopy: This method allows continuous measurement of the regional tissue saturation and oxygen extraction. It works non-invasively by placing a simple electrode on the forehead, kidneys, abdomen or muscles depending on the target organ. The electrode contains an emitter of infrared light at specific wavelengths and a receptor measuring the regional O2 saturation based on the amount of light absorbed by the hemoglobin. The method is comparable to arterial SpO2 monitoring. However, while the measurements of arterial SpO2 are performed about 2200 times/min, allowing the machine to capture SpO2 during the pulsations, near infrared spectroscopy (NIRS) emits an infrared light continuously, thus providing a measurement of O2 saturation mainly at the venous side of the capillaries (70%). Therefore, NIRS is primarily a measurement of venous saturation and should be considered as a non-invasive alternative of ScVO2. The method has been validated extensively and its use was shown to improve outcomes[28]. New generations of NIRS are being developed to enable the assessment of cerebral autoregulation and to choose the appropriate target blood pressure within the autoregulation zone.
SUGGESTED THERAPY GUIDED BY HEMODYNAMIC MONITORING
Considering that BP = CO × SVR, measuring BP, CI and SVRI is recommended as it helps to select the most appropriate therapy based on the different combinations of these parameters (Table 2). For example, a patient with a high SVRI, low CI and normal BP is better treated with inodilators such as milrinone or levosimendan to increase cardiac contractility and decrease afterload. Frequent assessments are required until the target clinical and laboratory hemodynamic parameters are reached, and CI and SVRI normalized. On the other hand, if SVRI, CI and BP are all low, norepinephrine is a first choice as it increases the SVRI, to be then followed by epinephrine for its beta 1 effect or by dobutamine if the CI remains low. Frequent assessments are always required until the hemodynamic targets are reached. In cases of low SVRI, high CI and low BP fluid boluses are administered first, followed by norepinephrine, then by low dose vasopressin if needed. As these potent vasoconstrictors can decrease CO, inotropes like dobutamine or low dose epinephrine can be added to improve cardiac contractility[29]. The whole situation needs close, frequent monitoring by clinical and laboratory assessment, in addition to these parameters. They could also guide the titration and the choice of these cardiovascular medications.
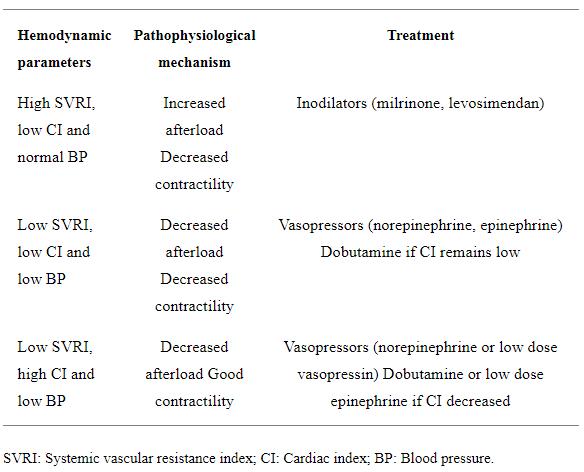
Table 2. Suggested therapy in response to measured hemodynamic parameters in children with septic shock
CONCLUSION
Early hemodynamic management in the first golden hour of presentation of septic shock is associated with better outcome[30]. Non-invasive bedside assessment of the preload, contractility and afterload provides a rational guide to the fluid therapy and selection of appropriate cardiovascular medications. Following the trend of hemodynamic parameters instead of individual readings allows for personalized management and proper titration of therapy over time. Proper training and a good understanding of the used techniques and their limitations is needed. With a paucity of existing data analyzing the effect of non-invasive bedside hemodynamic monitoring on mortality in children with septic shock, adequately designed and powered outcome studies are urgently needed.
ACKNOWLEDGMENTS
Dr. Samah Al Asrawi for providing the echocardiography picture which illustrates the measurement of the stroke volume.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest. No financial support.
Manuscript source: Unsolicited manuscript
Peer-review started: March 6, 2018
First decision: April 4, 2018
Article in press: May 11, 2018
Specialty type: Medical laboratory technology
Country of origin: United Arab Emirates
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Biltagi M, Losano G S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
Contributor Information
Emad Mohamed Fathi, Department of Critical Care, Al Jalila Children’s Specialty Hospital, Dubai 7662, United Arab Emirates. ea.hcja@afatsuomfe.
Hassib Narchi, Department of Pediatrics, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain 17666, United Arab Emirates.
Fares Chedid, Neonatal Intensive Care Unit, Oasis Hospital, Al Ain 1016, United Arab Emirates.
References
1. Tibby SM, Hatherill M, Marsh MJ, Murdoch IA. Clinicians’ abilities to estimate cardiac index in ventilated children and infants. Arch Dis Child. 1997;77:516–518. [PMC free article] [PubMed]
2. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. [PubMed]
3. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. [PubMed]
4. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. [PubMed]
5. Ranjit S, Aram G, Kissoon N, Ali MK, Natraj R, Shresti S, Jayakumar I, Gandhi D. Multimodal monitoring for hemodynamic categorization and management of pediatric septic shock: a pilot observational study*. Pediatr Crit Care Med. 2014;15:e17–e26. [PubMed]
6. Wong HR, Dalton HJ. The PICU perspective on monitoring hemodynamics and oxygen transport. Pediatr Crit Care Med. 2011;12:S66–S68. [PMC free article] [PubMed]
7. Ceneviva G, Paschall JA, Maffei F, Carcillo JA. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics. 1998;102:e19. [PubMed]
8. Pollack MM, Fields AI, Ruttimann UE. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit Care Med. 1985;13:454–459. [PubMed]
9. Mercier JC, Beaufils F, Hartmann JF, Azéma D. Hemodynamic patterns of meningococcal shock in children. Crit Care Med. 1988;16:27–33. [PubMed]
10. Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, Okhuysen-Cawley RS, Relvas MS, Rozenfeld RA, Skippen PW, et al. American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock. Crit Care Med. 2017;45:1061–1093. [PubMed]
11. Cariou A, Pinsky MR, Monchi M, Laurent I, Vinsonneau C, Chiche JD, Charpentier J, Dhainaut JF. Is myocardial adrenergic responsiveness depressed in human septic shock? Intensive Care Med. 2008;34:917–922. [PubMed]
12. Barbier C, Loubières Y, Schmit C, Hayon J, Ricôme JL, Jardin F, Vieillard-Baron A. Respiratory changes in inferior vena cava diameter are helpful in predicting fluid responsiveness in ventilated septic patients. Intensive Care Med. 2004;30:1740–1746. [PubMed]
13. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40:845–853. [PubMed]
14. Lemson J, Nusmeier A, van der Hoeven JG. Advanced hemodynamic monitoring in critically ill children. Pediatrics. 2011;128:560–571. [PubMed]
15. Parr GV, Blackstone EH, Kirklin JW. Cardiac performance and mortality early after intracardiac surgery in infants and young children. Circulation. 1975;51:867–874. [PubMed]
16. Nidorf SM, Picard MH, Triulzi MO, Thomas JD, Newell J, King ME, Weyman AE. New perspectives in the assessment of cardiac chamber dimensions during development and adulthood. J Am Coll Cardiol. 1992;19:983–988. [PubMed]
17. Abbas SM, Hill AG. Systematic review of the literature for the use of oesophageal Doppler monitor for fluid replacement in major abdominal surgery. Anaesthesia. 2008;63:44–51. [PubMed]
18. Knirsch W, Kretschmar O, Tomaske M, Stutz K, Nagdyman N, Balmer C, Schmitz A, Béttex D, Berger F, Bauersfeld U, et al. Cardiac output measurement in children: comparison of the Ultrasound Cardiac Output Monitor with thermodilution cardiac output measurement. Intensive Care Med. 2008;34:1060–1064. [PubMed]
19. Beltramo F, Menteer J, Razavi A, Khemani RG, Szmuszkovicz J, Newth CJ, Ross PA. Validation of an Ultrasound Cardiac Output Monitor as a Bedside Tool for Pediatric Patients. Pediatr Cardiol. 2016;37:177–183. [PubMed]
20. Bernstein DP, Osypka MJ. Anonymous Apparatus and method for determining an approximation of the stroke volume and the cardiac output of the heart. United States patent US6511438B2. 2003:Jan 28.
21. Noori S, Drabu B, Soleymani S, Seri I. Continuous non-invasive cardiac output measurements in the neonate by electrical velocimetry: a comparison with echocardiography. Arch Dis Child Fetal Neonatal Ed. 2012;97:F340–F343. [PubMed]
22. Norozi K, Beck C, Osthaus WA, Wille I, Wessel A, Bertram H. Electrical velocimetry for measuring cardiac output in children with congenital heart disease. Br J Anaesth. 2008;100:88–94. [PubMed]
23. Zoremba N, Bickenbach J, Krauss B, Rossaint R, Kuhlen R, Schälte G. Comparison of electrical velocimetry and thermodilution techniques for the measurement of cardiac output. Acta Anaesthesiol Scand. 2007;51:1314–1319. [PubMed]
24. Suehiro K, Joosten A, Murphy LS, Desebbe O, Alexander B, Kim SH, Cannesson M. Accuracy and precision of minimally-invasive cardiac output monitoring in children: a systematic review and meta-analysis. J Clin Monit Comput. 2016;30:603–620. [PubMed]
25. Suttner S, Schöllhorn T, Boldt J, Mayer J, Röhm KD, Lang K, Piper SN. Noninvasive assessment of cardiac output using thoracic electrical bioimpedance in hemodynamically stable and unstable patients after cardiac surgery: a comparison with pulmonary artery thermodilution. Intensive Care Med. 2006;32:2053–2058. [PubMed]
26. Song R, Rich W, Kim JH, Finer NN, Katheria AC. The use of electrical cardiometry for continuous cardiac output monitoring in preterm neonates: a validation study. Am J Perinatol. 2014;31:1105–1110. [PubMed]
27. Kupersztych-Hagege E, Teboul JL, Artigas A, Talbot A, Sabatier C, Richard C, Monnet X. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111:961–966. [PubMed]
28. Plomgaard AM, van Oeveren W, Petersen TH, Alderliesten T, Austin T, van Bel F, Benders M, Claris O, Dempsey E, Franz A, et al. The SafeBoosC II randomized trial: treatment guided by near-infrared spectroscopy reduces cerebral hypoxia without changing early biomarkers of brain injury. Pediatr Res. 2016;79:528–535. [PMC free article] [PubMed]
29. Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666–688. [PMC free article] [PubMed]
30. Funk D, Sebat F, Kumar A. A systems approach to the early recognition and rapid administration of best practice therapy in sepsis and septic shock. Curr Opin Crit Care. 2009;15:301–307. [PubMed]